Sponsored Content by TakedaSep 21 2020
An interview with Dr. Carmen Escuriola-Ettingshausen MD, Hämophilie-Zentrum Rhein Main, Mörfelden-Walldorf, Germany on providing personalized care for bleeding disorders such as hemophilia and von Willebrand disease.
Please give an overview of prevalent bleeding disorders such as hemophilia and von Willebrand disease.
In patients with bleeding disorders, the clotting process does not work properly and as a result, they bleed for longer but also bleed into internal organs and joints, which might lead to debilitating injuries and may become life-threatening.
Hemophilia is an x-chromosomal inherited disease that primarily affects males and has two variations; hemophilia A and hemophilia B. Hemophilia A is characterized by a deficiency of factor VIII, whereas, in hemophilia B, the clotting factor IX is decreased.
What is very important, is that hemophilia A is a rare disease occurring in about 25 cases out of 100,000 male births. Hemophilia B is much rarer, occurring in about 5 out of 100,000 male births. We also have to distinguish between the different severities and, in general, we can say the more severe the factor VIII or factor IX deficiency is, the more severe the clinical phenotype is, and therefore, the more the patient bleeds.
If a patient has no spurs or only spurs, this can mean less than 1% residual activity of factor VIII, or factor IX. Whereas a normal residual activity is more than 70%. Therefore, lacking factor VIII or IX can lead to severe hemophilia.
Von Willebrand disease is also a genetic bleeding disorder. Some patients suffer from an acquired von Willebrand disease, but I would like to focus on the inherited form.
This is a disease that affects the primary, as well as the secondary, hemostasis. Therefore, it affects platelet function. Platelet aggregation (normal platelet function), is extremely important in forming the primary clot. In the second step, this clot is formed by fibrin production which is disturbed in patients with von Willebrand disease.
Because of this, these patients usually have the problem of mucocutaneous mucosal bleeds, such as nose bleeds and bleeds after surgical intervention, particularly in the mucosal area. Women usually have menorrhagia.
In contrast to hemophilia, this disease affects males as well as females, and, in its mild form, it is a more frequent bleeding disorder occurring in up to 1% of the whole population.
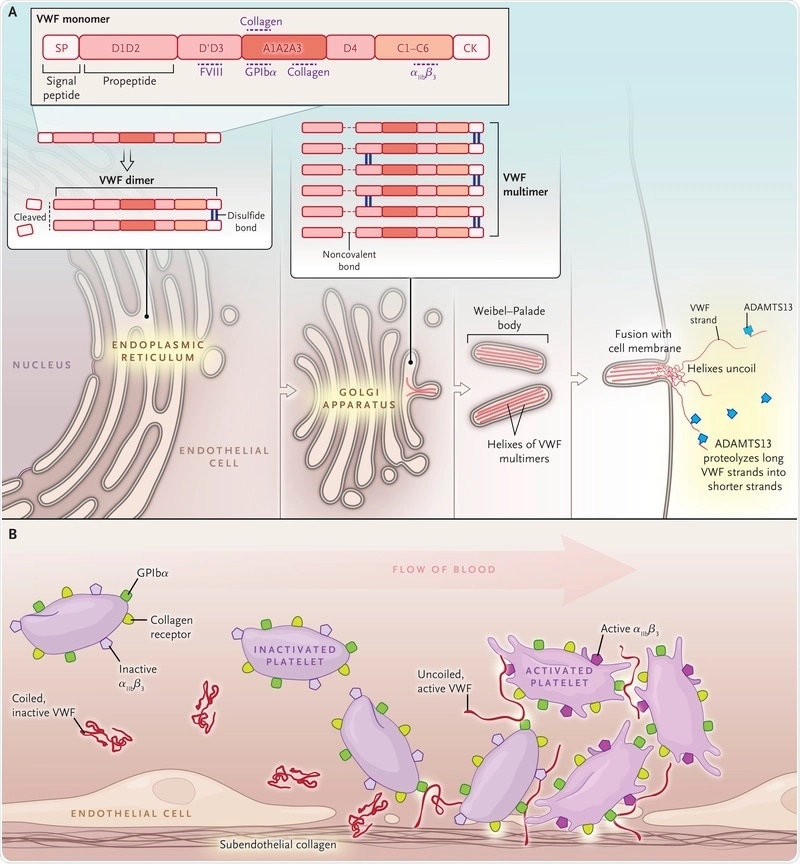
Image Credit: https://www.nejm.org/doi/10.1056/NEJMra1601561
What challenges are there for treating patients with bleeding disorders? Why can it be difficult to provide a ‘one size fits all’ treatment?
As hemophilia and general hemorrhagic diseases are rare disorders, the first challenge is to diagnose these diseases to offer adequate therapy, and not misdiagnose.
If a patient has been diagnosed, they will then need comprehensive treatment, which is personalized. This treatment can only be offered in dedicated and comprehensive care centers, where there are multidisciplinary teams who are experienced with the diagnosis, monitoring, and treatment of these patients.
We also must face the fact that those patients with chronic diseases are getting older and developing concomitant diseases, which have to be taken into account as well. I believe that access to diagnosis and, more importantly, access to treatment is extremely important.
In developed countries, patients do have sufficient treatment and access to comprehensive treatment centers, but from a worldwide perspective, we have to say that about 75% of people with hemophilia do not receive adequate treatment, and this is really a challenge.
What types of personalized treatments are available for patients with hemophilia, von Willebrand disease, and other bleeding disorders?
The cause of hemophilia and von Willebrand disease is lacking or decreased activity of either factor VIII, IX, or the von Willebrand factor. Therefore, the usual therapeutic approach is to replace the lacking factor.
However, we must distinguish from different situations of treatment for different patients. For example, if a patient has a moderate or mild disease, the clotting factor concentrates are replacing the decreased factor in case of bleeds or during surgery. This is called on-demand treatment.
However, if we focus on the severely affected hemophiliacs and also on those with type three von Willebrand disease (the most severe form of von Willebrand disease), they are bleeding so frequently, particularly into the joints which can lead to the development of arthropathy, and what we want to do is to prevent the bleeds and prevent the sequela.
We also want to prevent other severe bleeds, predominantly life-threatening bleeds, and this can be done with prophylactic therapy, which is a prophylactic administration of clotting factor concentrates at regular intervals. Prophylaxis has been introduced in the treatment of hemophilia for many decades now and there is good evidence that prophylaxis can prevent or reduce bleeds depending on when the prophylaxis has been started in the life of a hemophiliac.
However, we must consider that when we are using a standard regimen, we are not able to prevent as many bleeds as we would like. What we must consider is that each patient is different, and their pharmacokinetic properties can vary. There is a huge difference whether a patient has a factor VIII with a half-life of 6 hours or 18 hours. We have such a large span between the patients and also, an age-dependent one. Usually, young children have a shorter half-life of factor VIII than older people.
This is not true for the factor IX, however. Here we do not see such a variation.
Nevertheless, we have to take this and many other variables (joint status, bleeding phenotype, physical activity, etc.) into account and create the right therapy for the right patient in the right situation. This is called the personalization of prophylaxis.
So, which therapeutic agents can we use? Can we use the standard half-life concentrate? Let's say these are the standard products, which we have had for on-demand treatment, as well as for prophylactic treatment, for many years. The half-life is more or less the same as the half-life in healthy people (usually in between 8 and 14 hours), and you can imagine these patients have to receive very frequent infusions, usually, 3 times weekly, to maintain a factor VIII level above 1%, which converts them from a severe to a moderate hemophiliac.
But technology does not stand still, and during the last few years concentrates containing clotting factor with an extended half-life have been developed and come onto the market. Half-life extension succeeded in hemophilia B products (factor IX). They have an up to five-fold half-life extension, leading to less frequent prophylactic injections (from once a week up to once every three weeks).
In hemophilia A, this is a little bit different. Half-life extensions have also succeeded: We now have a half-life extension of around 1.5-fold, compared to the standard half-life products. However, I think this prolongation is extremely important because we have two options with this; in some patients who are not as active, we can extend the administration interval. So, instead of the three times weekly, patients may inject twice weekly, which is a relief for the burden of treatment of all these patients.
On the other hand, we also have the possibility to inject these concentrates in the same frequency as those with a standard half-life (three times weekly), and we can achieve elevated trough levels, offering more protection for a patient. I think this is extremely important, at least for a subset of patients in a certain period of their life when they require more protection.
In addition, other therapies came on the market, such as non-replacement therapies. These are monoclonal antibodies, which mimic the actions of factor VIII. Other non-replacement therapies are under development. This is also true for gene therapy. The goal of gene therapy is to offer a patient a sustained therapeutic level of their clotting factor over multiple years.
Gene transfer is done by the missing gene being packaged into a delivery vehicle, known as a vector (usually adeno associated viral, AAV, vectors are used). The data is very promising. Nevertheless, this therapeutic approach will be available for adult patients in the very near future and still might be associated with some complications such as mild liver toxicities. The patient with pre-existing immunity against AAV antibodies are also not suitable candidates for gene therapy.
Image Credit: Kateryna Kon/Shutterstock.com
How can prophylaxis be pharmacokinetically guided? What are the advantages and limitations of using this technique?
The knowledge of individual pharmacokinetic properties is extremely important to tailor the adequate prophylactic regimen for the hemophilia A patient. But, in addition to the patient’s pharmacokinetic profile, we have to include all other individual factors that might have an impact on the creation of this individual therapeutic regimen, such as the clinical phenotype, joint status, venous access, the willingness to perform frequent or less frequent injections, and, of course, the pharmacokinetic background of this patient.
How do we perform pharmacokinetic evaluations? I think people who are not close to hemophilia think, "Okay. Let's do a classical pharmacokinetic approach." This means that the patient needs a washout phase, followed by the injection of the concentrate. Thereafter frequent blood samples over a certain period are taken to assess factor VIII activity.
Another option is population pharmacokinetic modeling. Here we do not need a washout phase, so the patient is fully protected. Secondly, we only need a few time points for blood sampling. Usually, two samples are sufficient to determine a valid pharmacokinetic profile for a patient. The calculation of factor VIII half-lives can be done using different platforms/calculators, such as myPKFiT (product dependent), WAPPS-Hemo, and Florio. After assessing the factor VIII half-life we can model individual prophylactic treatment regimens, adapted according to the patient´s needs.
Most of these platforms also offer a mobile application so that patients are able to document their clotting factor therapy and see their estimated factor rate levels over time.
This way, the patient can be actively involved in the treatment and has a more protective infusion regimen adapted according to physical activity.
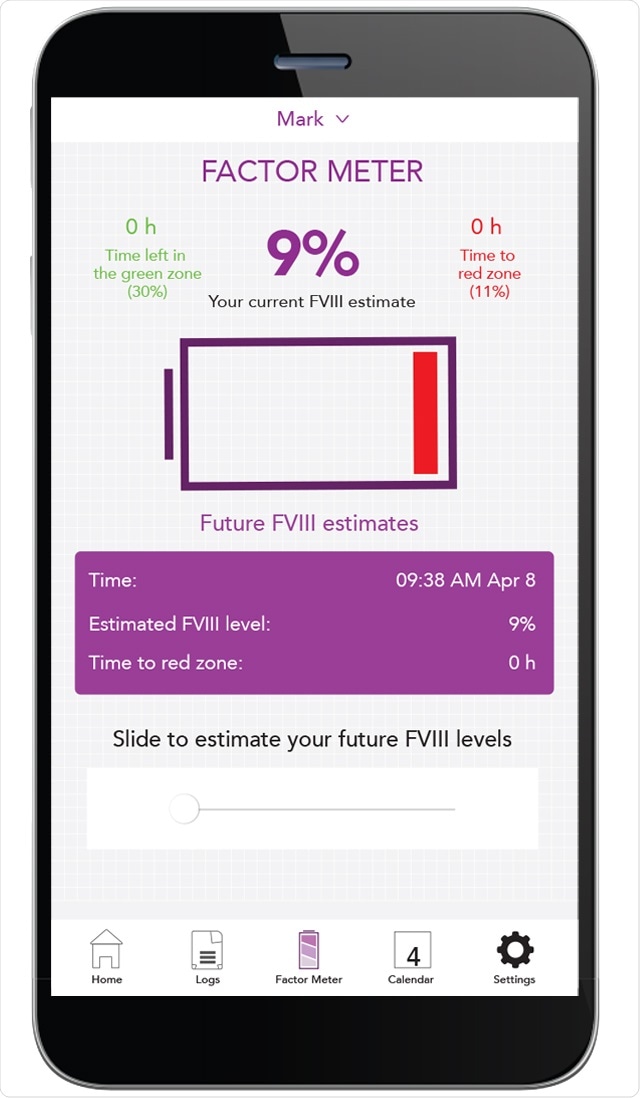
Image Credit: https://www.personalizedpk.ca/en/about.html
Please describe your research in the CONTINUATION and PROPEL studies, with updates recently presented at the International Society on Thrombosis and Hemostasis (ISTH) 2020 Virtual Congress.
I was one of the investigators of the PROPEL study looking at personalizing prophylaxis and the possibility to elevate trough levels of factor VIII or to prolong dosing intervals with the use of extended half-life products in patients with hemophilia A.
Since we had no clinical data from studies supporting the theory that higher trough levels are superior, or a higher trough level protects patients better, this was one of the main objectives of the PROPEL study.
In the PROPEL study, 115 patients, older than 12 years, with severe hemophilia A, who have been pre-treated numerous times with clotting factor concentrates, have been randomized to a prophylactic therapy regimen targeting the classical factor VIII trough level, which is 1% to 3%, versus a more elevated trough level, namely, 8% to 12%.
How did they achieve this? All patients had to undergo pharmacokinetic investigation in order to create their individual therapeutic regimen to achieve these different trough levels. Before and during the study phase bleeds in their target joints, spontaneous bleeds, and annual bleed rates were assessed.
What was really remarkable was that those patients who had higher trough levels clearly benefited from them which means markedly reduced bleeding into target joints and a markedly reduced annual bleed rate, within the first six months of this therapy.
Another cohort came from the CONTINUATION study. Those patients had the opportunity to perform pharmacokinetic-guided prophylaxis or continue with their twice-weekly prophylactic therapeutic approach. What could be observed is that those patients also benefited from the individualization of therapy.
I think it is extremely important to show that elevating the trough level really is a benefit for patients with severe hemophilia A because it is more protective, and it also has the possibility to reduce or declassify already existing target joints.
How has your research been impacted by your long-term partnership with Takeda? What are your experiences working with the research department at Takeda?
There are many extremely experienced people working at Takeda and creating studies, such as the PROPEL study, working on the development of new treatment strategies, but also new treatment options and new therapies in order to improve the lives of patients with hemophilia and von Willebrand disease.
Takeda is the company that developed new treatments for patients with bleeding disorders, such as factor VIII concentrates with extended life-life or a recombinant von Willebrand factor. Well established and safe concentrates for the treatment of hemophilia with and without inhibitors are solid treatment options for many years. So, there is a very long partnership and the experience was excellent. There is a strong exchange in-between doctors, treatment centers, and Takeda.
What effect do you hope this research will have for patients who live with these bleeding disorders?
I think research is mandatory to improve the life of patients in general. Preventing bleeds and the sequela (joint disease) should be our aim to preserve the joint health and to enable them to participate in social activities.
I think, at the moment, where we do not have an entirely healing therapy for those patients, we should do our very best to offer the patient the best therapeutic approach, and I think for the complete cohort of severe hemophilia, this is personalized prophylaxis.
Of course, personalized prophylaxis usually aims to prevent all bleeds. But from a world-wide perspective, personalization of therapy can also be implemented with a lower amount of resources, for example in developing countries.
Image Credit: Jarun Ontakrai/Shutterstock.com
In these challenging times, bleeding disorder patients have had to adapt to a new normal, including working from home and shielding to protect from COVID-19. How can personalized treatment be used to help support these people?
We have the technical requirements that patients can be closely connected to our centers, allowing us to use telemedicine. We can consult our patients through virtual consultations and we can also monitor their infusion therapies remotely.
But using telemedicine has its clear limitations, for example, we cannot perform any blood sampling or physical examination.
However, using online platforms and patient apps such as MyPKFit offers good monitoring of the patient. For patients using those devices actively, treatment can be adapted according to the patient´s needs. In case of bleeding events, the treating physician is immediately informed and can support treatment strategies at home.
What is the future of personalized treatment for bleeding disorders?
In the future, all patients will have a personalized treatment. This means that the different treatment options we have or are going to have in the near future will be implemented according to the patient´s demands during their whole lifespan.
I think patients will have the opportunity to be treated by different treatment options, for example, starting with replacement therapy, bridging to a non-replacement therapy, then potentially have a gene therapy, followed by another phase of replacement therapy. I think the choice and the intensity of treatment will also be more personalized over the lifespan of a patient.
About Dr. Carmen Escuriola-Ettingshausen, MD
Dr. Carmen Escuriola Ettingshausen is the Director of the Hämophilie-Zentrum Rhein Main - HZRM, Frankfurt-Mörfelden, Germany.
She graduated in medicine at the Johann Wolfgang Goethe-University in Frankfurt from 1985 to 1992. After that, she became a resident at the comprehensive care center for Thrombosis and Haemostasis of the Children´s Hospital at University Hospital of Frankfurt in the department of Pediatrics until 1996 and obtained the Doctor title in 1995.
She performed specialist´s training in Pediatrics at the University Hospital of Frankfurt from 1996 until 2000, and since then she was a staff member at the comprehensive care center for Thrombosis and Hemostasis of the University Hospital of Frankfurt, Department of Pediatrics until 2012. In 2012 she founded the Hämophilie-Zentrum Rhein-Main - HZRM, Frankfurt-Mörfelden.
Dr. Escuriola-Ettingshausen’s clinical interests include hemorrhagic disorders with a focus on hemophilia, thrombosis, congenital immunodeficiencies, and hereditary angioedema in both pediatric and adult patients.
Her research interests are hemorrhagic disorders, particularly in the treatment of hemophiliacs and hemophiliacs with inhibitors. Moreover, she is a member of the German, Swiss, and Austrian Society For Thrombosis and Haemostasis Research (GTH), International Society for Thrombosis and Haemostasis (ISTH), and the International Prophylaxis Study Group (IPSG).