Researchers from Clover Biopharmaceuticals, the Chinese Academy of Sciences, and China’s National Institutes for Food and Drug Control (NIFDC) report a trimeric subunit vaccine candidate similar to the spike protein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The vaccine candidate produced a high level of neutralizing antibodies in animal models. Rhesus macaques immunized with the vaccine candidate showed reduced viral loads in the lungs. The research is published on the preprint server bioRxiv*.
One of the most important strategies for preventing the COVID-19 pandemic from spreading is the development of vaccines. Any future SARS-CoV-2 vaccine needs to be effective, safe, and easy to develop and manufacture.
Some of the types of vaccines being developed for COVID-19 include mRNA and DNA vaccines, inactivated SARS-CoV-2 virus, and adenosine-based viral vectors. Many candidates are in different stages of clinical trials.
A major challenge in developing an effective vaccine for an RNA virus is the difficulty in inducing broadly neutralizing antibodies, such as seen with HIV vaccines. Also, with viruses that affect the respiratory system, like SARS-CoV-2, there is a risk of vaccine-associated enhanced respiratory disease.
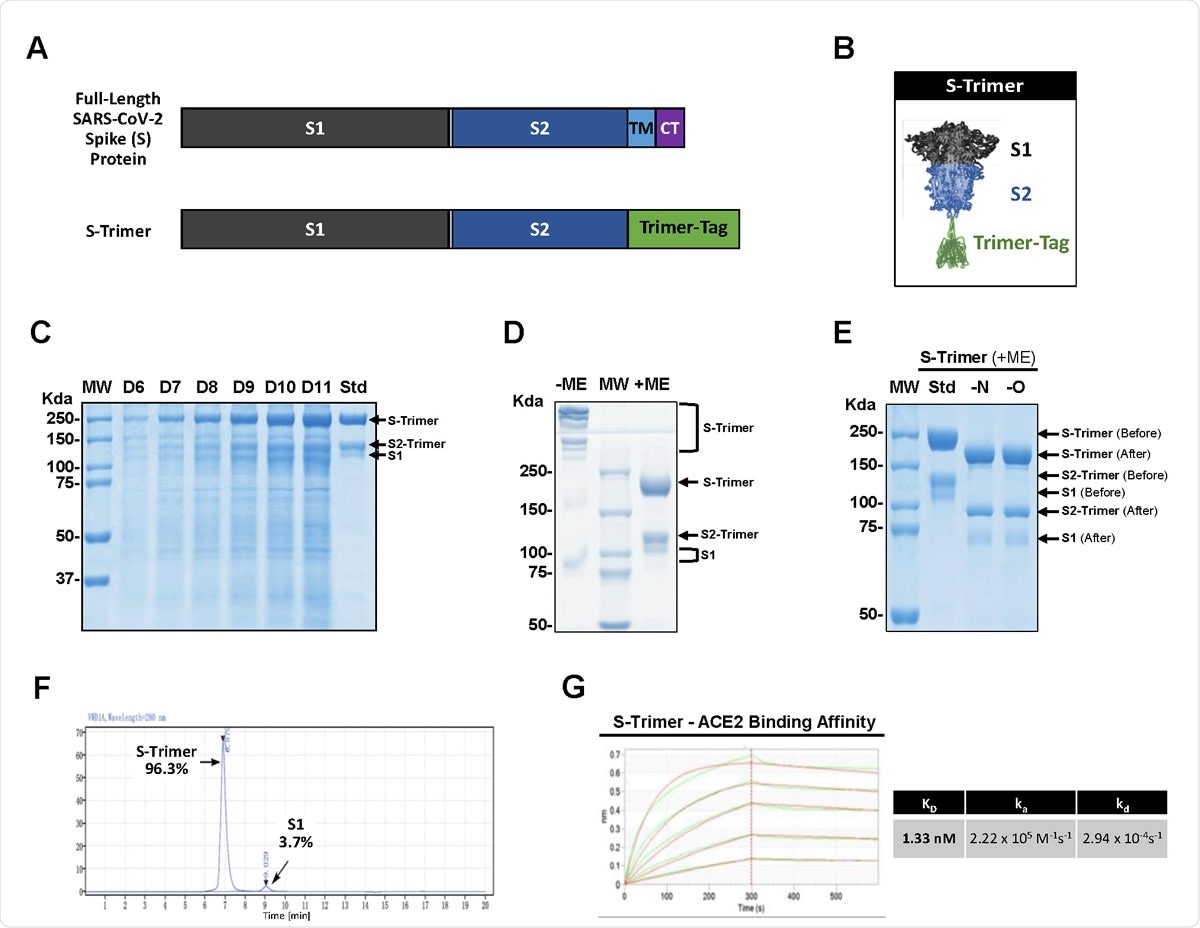
High-level expression and Characterization of S-Trimer. (A) Schematic representations of full-length SARS-CoV-2 Spike (S) protein and the ectodomain of wild-type SARS-CoV-2 S protein-Trimer-Tag fusion protein (S-Trimer). (B) Schematic 2-D illustration of S-Trimer with homotrimeric Spike protein in the prefusion conformation. (C) Reducing SDS-PAGE analysis with Coomassie Blue staining of high-level expression of S-Trimer as a secreted protein from CHO cells in a 15L bioreactor Fed-batch serum-free culture over 11 days (10 μL of cleared media were loaded for each sample) along with a purified standard (Std). (D) S-Trimer is a disulfide bond-linked homo-trimer as analyzed by SDS-PAGE with Coomassie Blue staining under non-reducing (-ME) and reducing (+ME) conditions. S-Trimer was shown to be partially cleaved at S1/S2 junction as indicated. (E) S-Trimer is heavily N-glycosylated. Analysis of S-Trimer before and after deglycosylation with PNGase F (-N) and PNGase F & Endo-O (-O) by SDS-PAGE with Coomassie Blue staining under reducing (+ME) condition. (F) SEC-HPLC analysis of the purity of S-Trimer with an MW of approximately 700 Kda, and a small fraction of cleaved S1 was shown detached from S-Trimer as indicated. (G) Determination of the binding affinity between S-Trimer and human ACE2-Fc by ForteBio BioLayer interferometry.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Trimeric protein like SARS-CoV-2 spike protein
The SARS-CoV-2 virus binds to host cells using the trimeric spike protein. This trimeric antigen binds to angiotensin-converting enzyme 2 (ACE2) produced by the host cells, helping the virus gain entry into the host cell.
In a new report, researchers report a technology to produce a trimeric protein, S-Trimer, like the spike protein in the virus, which can produce antibodies upon infection in host cells.
To produce the S-Trimer, the researchers used Trimer-Tag technology, which was reported previously. Using this technology, they produced clones of the SARS-CoV-2 spike protein using cDNA after transfection into CHO cells, which self-trimerized via disulfide bonds. They purified the S-Trimer protein using the high affinity of Trimer-Tag to Endo180, a collagen receptor.
The S-Trimer vaccine candidate has the complete sequence of the wild-type SARS-CoV-2 spike protein and has a high affinity to the ACE2 receptor. The authors write that their purification scheme for the S-Trimer, producing 500 mg/L, can allow several billion doses to be produced annually in 2000 L bioreactors.
Next, the scientists used the S-Trimer to detect if antibodies to SARS-CoV-2 spike proteins were present in the sera of 41 patients who recovered after COVID-19. They found a high level of antibodies that bound to S-Trimer.
They also found a higher level of antibodies in patients with severe disease and lower levels in patients with mild disease. However, many of the patients, especially with mild disease, did not have any antibodies binding to ACE2, suggesting antibodies may target not only the receptor-binding domains (RBDs) but also other domains of the virus.
This suggests that patients who develop a rapid T-cell response may not need high levels of antibodies to neutralize the virus. It has been reported before that patients with mild disease develop strong T-cell immunity, while patients with severe disease have very low T-cells.
Immunity in monkeys
The authors first tested the immune response to S-Trimer in mice. When used with an adjuvant, like AS03 and CpG 1018 with alum, the levels of both pseudovirus neutralizing and ACE2-competitive antibodies were similar to or more than those in human sera.
Next, they administered the S-Trimer with adjuvants to rhesus macaques to study the immune response. After two doses of the vaccine candidate, 21 days apart, the animals were exposed to SARS-CoV-2 35 days after the first dose.
The macaques showed high levels of both neutralizing and binding antibodies, with the number increasing after the second dose. Although CpG 1018 plus alum adjuvant showed lower antibody levels than human sera compared to that of AS03, the animals with the former adjuvant mounted a faster and more durable lymphocyte response, which remained high 7 days after infection.
The animals that did not receive the S-Trimer and adjuvants showed a rapid loss in body weight of about 8% in 7 days after infection, whereas the other animals did not lose weight, nor did they have a fever. The macaques that received doses of the vaccine candidate also showed low viral loads in lung tissues, throat, and nasal swabs.
Both AS03 and CpG 1018 plus alum adjuvants induced sufficient levels of protection against SARS-CoV-2 in monkeys, and there was no disease enhancement.
“Collectively, these results support the advancement of adjuvanted S-Trimer through human clinical studies to further demonstrate safety, immunogenicity, and vaccine efficacy,” write the authors.
The recombinant production of S-Trimer using Trimer Tag can also be rapidly scaled up, write the authors, and the vaccine can be stored at 2–8 °C, and does not need to be stored in freezers.
Phase I clinical trials of the vaccine candidate commenced in June 2020, with further phases planned.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references:
- Preliminary scientific report.
Liang G. J. et al. (2020) S-Trimer, a COVID-19 subunit vaccine candidate, induces protective immunity in nonhuman primates. bioRvix. https://doi.org/10.1101/2020.09.24.311027
- Peer reviewed and published scientific report.
Liang, Joshua G., Danmei Su, Tian-Zhang Song, Yilan Zeng, Weijin Huang, Jinhua Wu, Rong Xu, et al. 2021. “S-Trimer, a COVID-19 Subunit Vaccine Candidate, Induces Protective Immunity in Nonhuman Primates.” Nature Communications 12 (1). https://doi.org/10.1038/s41467-021-21634-1. https://www.nature.com/articles/s41467-021-21634-1.