Since the beginning of the COVID-19 pandemic, researchers have noted that women are generally less severely affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This has led to the exploration into estrogen and androgens' role in the hyperinflammatory clinical phenotypes of COVID-19. A recent study published on the preprint server bioRxiv* in October 2020 shows that drugs targeting androgens and TMPRSS2 inhibitors may potentially combat the pandemic.
Males are more likely to incur the disease and are more often prone to severe disease and death due to COVID-19. Therefore, the most high-risk group includes older males with underlying chronic health conditions such as diabetes, cardiovascular conditions, obesity, and hypertension.
Inflammation and Severe Disease
The male predilection indicates that androgens play a key role in the susceptibility to SARS-CoV-2 infection and the host response. The latter is often observed to involve a cytokine storm, triggered by hyper-inflammatory responses to the infection.
Typically, cytokines and chemokines such as tumor necrosis factor-α (TNF- α), interleukin 1β (IL-1 β), interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein 1-α (MIP1α) are upregulated in this phase, which is correlated with severe and/or fatal COVID-19.
Tumor microenvironments are known to enhance tumor development and growth as well as progression. Since these are known to be rich in inflammatory cytokines, the researchers considered the possibility that the high levels of these factors in severe COVID-19 could accelerate cancer progression. Therefore, this could be an indication to develop specific interventions to treat this disease in cancer patients who are already in an immunosuppressed condition and so more likely to be infected.
Androgen-Mediated Differences in Entry Factors
Viral entry into the host cell is thought to be via the viral spike protein binding with the angiotensin-converting enzyme 2 (ACE2) receptor on target cells' surface. ACE2 is expressed on the heart, testis, kidney, and in other tissues.
This virus-ACE2 binding mediates viral internalization and infection. Host proteases such as TMPRSS2 are instrumental in enhancing the virus's entry by promoting viral-cell membrane fusion.
TMPRSS2 has undergone extensive study for its androgen regulating characteristics in prostate cancer since it drives abnormal oncogene expression. However, not much is known about how ACE2 interacts with this protease.
The current study is based on the need to understand the molecular-level interactions that determine the expression of TMPRSS2 and ACE2. This, in turn, could underlie the differences in infection susceptibility, the disease severity, and the death rate between men and women.
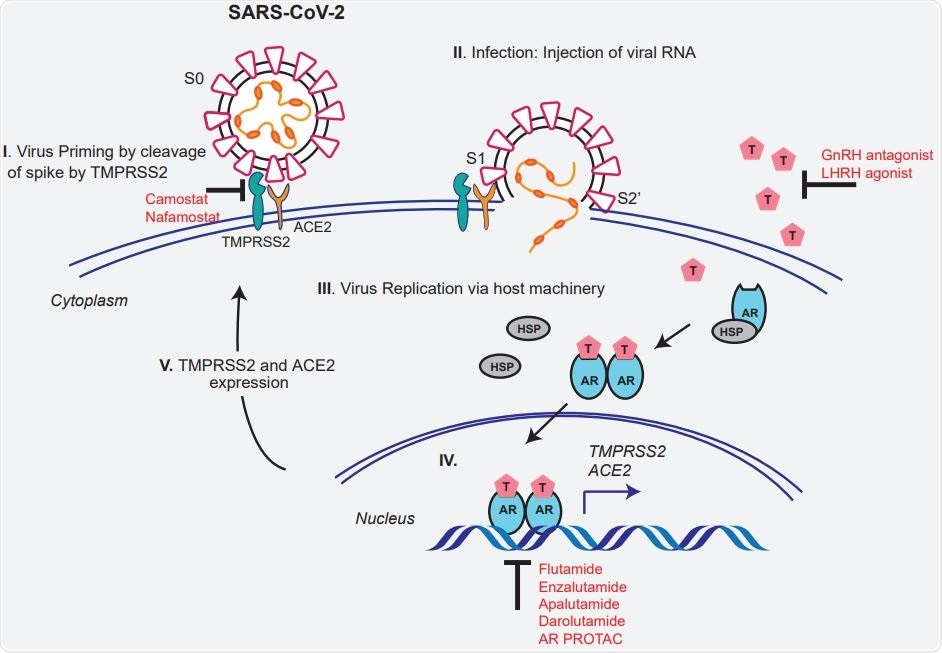
Schematic depicting the role of TMPRSS2 in SARS-CoV-2 Spike cleavage, and androgen-mediated expression of ACE2 and TMPRSS2 that could potentially be targeted by AR directed therapies.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Androgen Deprivation Affects TMPRSS2 and ACE2 expression in mice
Both AR and TMPRSS2 were expressed at the highest levels in mouse seminal vesicles, while the highest ACE2 expression was in the small intestine. This was repeated in human male tissue, which indicates the extrapolation of the mouse study to humans is a valid initial strategy.
Secondly, they found a strong correlation between TMPRSS2 and androgen receptor (AR) expression in the seminal vesicles after castration, unlike the other tissues. The hormone-responsive prostate cells had lower levels of AR and TMPRSS2 following castration in mice.
ACE2 expression in the seminal vesicles, lung, and small intestine was decreased after castration to levels approaching those in female mice. In the renal tissues, castration was followed by an increase in ACE2 levels in the kidney.
Since ACE2 is part of the renin-angiotensin-aldosterone system, of which the kidney is a vital part, castration, which reduced androgen levels and therefore the blood pressure, resulted in the downregulation of ACE2.
AR-Dependent Regulation of TMPRSS2 and ACE2 Expression
The researchers also found that AR were found to be closely involved in the initiation of TMPRSS2 and ACE2 transcription in the prostate tissue exposed to testosterone. These regions contained androgen response elements (ARE) and steroid binding sites, which indicates they are subject to regulation by steroids as well.
TMPRSS2 interacts with ACE2 in prostate and lung cells
They also found that both TMPRSS2 and ACE2 interact to produce their action. Specifically, TMPRSS2 produced ACE2 cleavage at the N-terminal end, which was necessary for SARS spike-ACE2 interaction. High TMPRSS2 expression in ACE2-expressing cells results in ACE2 cleavage.
This is blocked by TMPRSS2 inhibitor Camostat, which indicates that ACE2 is a substrate of the former enzyme. These experiments demonstrate that the ACE2 interacts on a physical level with the TMPRSS2 in lung and prostate cells. This endogenous complex does not appear to be dependent on TMPRSS2-mediated ACE2 cleavage.
TMPRSS2 Inhibition Blocks S Priming Independent of ACE2 Binding
Earlier researchers have suggested that the SARS-CoV-2 spike protein is cleaved by TMPRSS2, but the evidence is deficient. The current experiment included co-transfection of plasmids expressing the spike and the enzyme.
As expected from some recent studies, in this situation, the full-length spike was cleaved into S2 and S2’, with inhibition by Camostat. However, the latter failed to reduce the amount of ACE2 being pulled down by TMPRSS2.
The researchers suggest, “The SARS-2-S cleavage by TMPRSS2 is mediated in the presence of ACE2 in the complex.”
However, the pulldown does not contain any cleaved S0 fragments. This could mean the cleavage fragments are stable and released from the complex. Thus, TMPRSS2 is vital to mediating or promoting viral fusion to the host cell through the process of spike priming.
Camostat alone or in combination with AR directed therapies reduces SARS-CoV-2
The researchers looked at Camostat's efficacy and other anti-AR therapies to inhibit SARS-CoV-2 entry via spike protein binding. Camostat prevented viral entry only in androgen-deprived cells, indicating that TMPRSS2 could be a cofactor for its activity.
In androgen-proficient cells, Camostat, anti-androgen enzalutamide, or AR degrader ARD-69 all brought about an observable drop in viral entry. Still, the combination of Camostat with either of the others was more effective than monotherapy. Moreover, AR-negative cells showed reduced viral entry only with Camostat.
Implications
the study may help to understand the higher risk of COVID-19 posed to men. It shows that androgen receptors regulate both TMPRSS2 and ACE2. In vitro and mouse studies showed a reduction in androgen levels to be correlated with decreased TMPRSS2 and ACE2.
Again, androgen also reduces immune function while increasing the propensity to inflammation and may increase the severity of the disease in men. Androgen induces a rise in circulating neutrophil count and IL-8 (among other cytokines), which are characteristic of severe COVID-19.
If progressive COVID-19 is due to uncontrolled replication of SARS-CoV-2, the current study's findings may prove valuable since they show the importance of androgen effects on ACE2 and TMPRSS2 expression.
Androgens may act via TMPRSS2, which in turn enhances the uptake of the virus either by interaction with or cleavage of ACE2 or by spike cleavage, which enhances viral entry into the host cell. The study demonstrates how AR-expressing lung and prostate cells can be treated with a TMPRSS2 inhibitor and anti-AR drugs to inhibit viral entry via the spike protein.
This could indicate the potential for these drug combinations in preventing the progression of COVID-19 in men, with or without prostate cancer.
The researchers say, “We provide the first direct evidence for the endogenous interaction between TMPRSS2 and ACE2 in human cells, and endogenous TMPRSS2 mediated cleavage of SARS-CoV-2 Spike, which could be blocked by Camostat.” This agrees with prior research showing how TMPRSS2 contributes to the activation of the spike protein of SARS-CoV and MERS-CoV.
Clinical trials are already underway, testing the effects of anti-androgen and androgen deprivation therapy. This study provides a mechanistic explanation for these therapies and Camostat, also under study as a potential drug for COVID-19 treatment. The latter could be combined with antivirals to help prevent severe SARS-CoV-2 infection in patients with advanced cancer who are already susceptible due to pre-existing immunosuppression and other health impairments.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources