Adding another piece to the puzzle that is coronavirus disease 2019 (COVID-19) serology, a new study published on the preprint server medRxiv* in November 2020 suggests that males have stronger but more transient anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) protective immunity than females.
Humoral immunity to SARS-CoV-2 is still a topic of intensive debate. Data from the beginning of the pandemic shows that males, the elderly and those with a higher body mass index have a greater risk of severe disease, and therefore of higher antibody production compared to asymptomatic or mild infections.
Some earlier studies have shown that antibody titers remain stable over the first three months post-infection, but others report a rapid decline in antibodies over time, whether the disease is mild or severe. Most researchers consider the anti-spike antibody to be synonymous with virus-neutralizing capacity, with S being the major, if not unique, target for neutralizing antibodies.
Variation in the titer of neutralizing antibodies differs with the time since the onset of symptoms as well as disease severity, but not much is clear as to how other factors, including sex, age and body mass index, affect immunity. This is especially pertinent for mildly infected people, who make up the majority of COVID-19 infections.
The current study reports the results of a single-center antibody analysis, on about 300 cases, all confirmed by real-time polymerase chain reaction reverse transcription (RT-PCR) testing. All subjects were working in Strasbourg University Hospitals as doctors, nurses, caregivers and other administrative staff. About 75% were female, with a median age of 39 years.
Of the participants, 94% were symptomatic, albeit mildly, with symptoms like dry cough, fever, shortness of breath, anosmia and ageusia. Over a third had a history of contact with infected people outside or inside the hospital. None developed severe or critical illness, but 5% had moderate disease requiring hospitalization. Most developed symptoms within 1-5 days.
Testing for antibodies to viral antigens
Blood samples taken at a median of 31 days and 107 days from the onset of symptoms were used to estimate the rates of antibody positivity, using four separate assays. The S-Flow Assay was used to measure anti-S IgG, with 99% and 100% sensitivity and specificity, respectively. This showed antibodies to be present in 100% of cases at the first time point, M1, and only 3 became negative by the second time point, M3-6.
Secondly, they tested both IgG and IgM anti-Spike antibodies using a lateral flow assay with somewhat less sensitivity but more stability over time. This showed 85% seroprevalence of IgG antibodies at both time points. IgM was found in 93% of patients at M1 and around 80% at M3-6, which may indicate a decline in this antibody class over time.
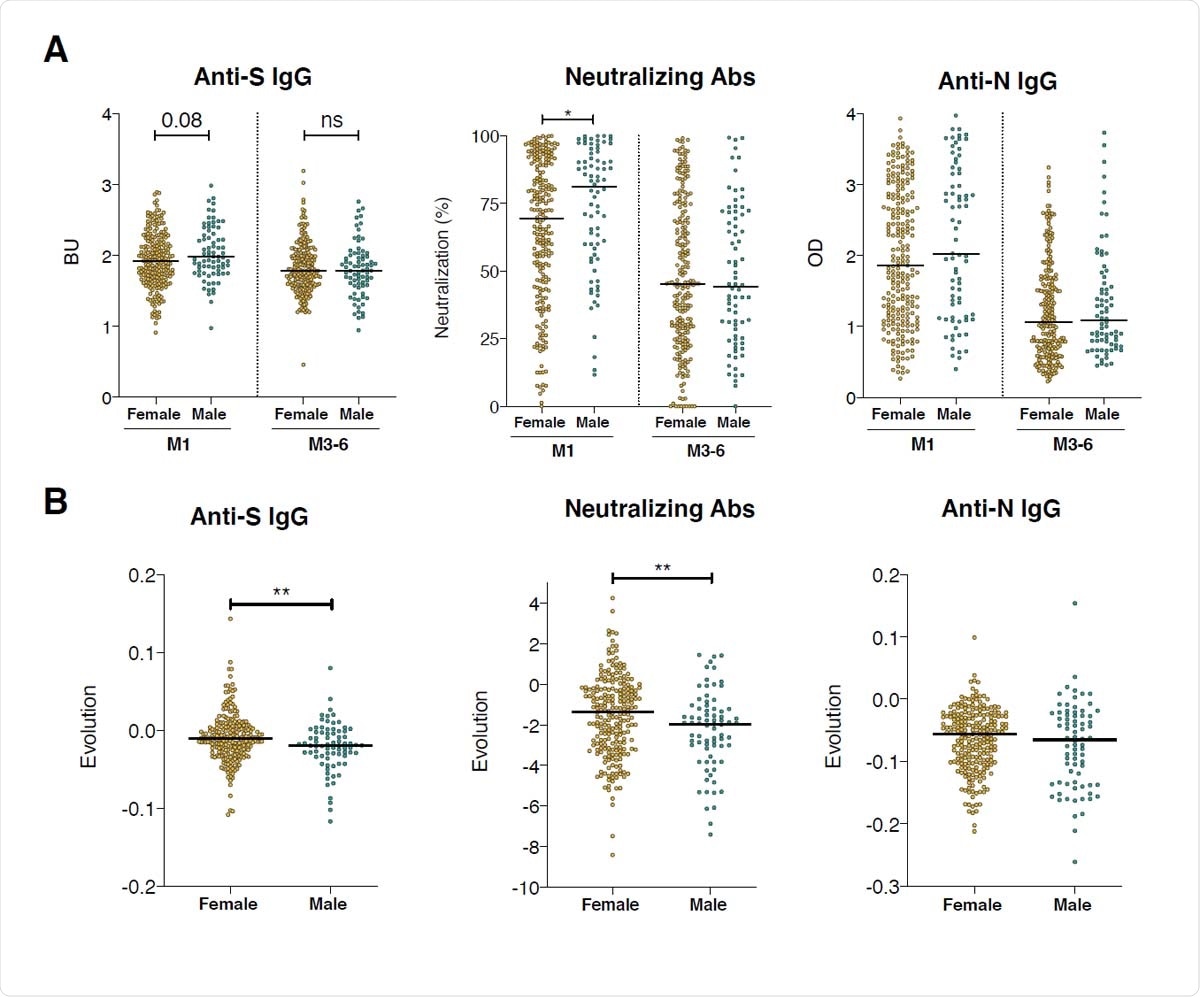
Sex differences in anti-SARS-CoV-2 antibody levels at the two samplings and their temporal evolution. A. Anti-S IgG (in BU), percentages of neutralization and anti-N IgG (in OD) were compared between males (green dots) and females (orange dots) at M1 or at M3-6 POS. The black line represents the median of all samples for each time-point. Samples from females and males were compared at each time-point with a Mann-Whitney test, * p<0.05, ns: not significant. p-value or non-significance (ns) are indicated on the segments. B. Weekly evolution of antibody levels between M1 and M3-6 was calculated as (level at M3-6 - levels at M1) / (# weeks POS M3-6 - #weeks POS M1). Color coding and graphical parameters are as in A. The dotted line represents a stable antibody level (evolution of 0). Statistical analysis Mann-Whitney test, **p<0.01.
The third test, an enzyme-linked immunosorbent assay (ELISA) assay targeting anti-N IgG, showed similar trends to the second at M1, but only around 60% were positive at M3-6. This could mean that mild infections elicit lower titers of anti-N, or just lower test sensitivity, or that the anti-N antibodies follow a different pattern. Test sensitivity is a time-dependent parameter, varying with the period since symptom onset.
Time-dependent decline in neutralizing antibody titer
The second part of the experiment measured the titers of neutralizing antibodies in serum, using one based on pseudotyped SARS-CoV-2 S-expressing lentivirus. They tested the serum at a non-saturating dilution of 1:100 to calculate the neutralizing activity, since the percentage-neutralization at this dilution corresponds well with that obtained by successive dilutions. Setting a threshold of 20% neutralization for a positive test, they found that 95% and 84% of sera were positive at the two-time points. If the threshold was raised to 50% or 80%, the neutralization activity was shown to decrease over time.
They found that anti-S and neutralizing antibodies were at higher levels in males at M1, as expected since males have a stronger initial immune response, including pro-inflammatory cytokines, than females. While both sexes showed a decline over time, the slope was gentler in females, so that by M3-6, both males and females had equivalent titers. No significant difference was seen with anti-N IgG at either time point.
The researchers also compared antibody titers at different time points to identify changing patterns. For this purpose, they used a Binding Unit (BU) based on a fluorescent signal established with a reference human anti-S monoclonal antibody. When the trend over time was examined, they found a small but significant downward movement of anti-S IgG, which was confirmed by a plot of the median values of anti-S, anti-N and neutralizing antibodies over time.
They found that while the majority of individuals (“decayers”) showed declining antibody titers, a few were “sustainers” with stable antibody titers. Of the sustainers, more were females.
About 70-83% of subjects were decayers, by any of the first three assays, with a median antibody half-life of 41 weeks for anti-S IgG. The neutralizing antibody titer dropped to half in around 20 weeks, and by around 18 weeks with anti-N IgG.
Other factors that influence antibody titer
When classified by age, older and heavier subjects had higher antibody titers at M1 for all three antibody types, as did those hospitalized with moderate disease. However, the higher the antibody titer at M1, the faster the decrease over the 172 days of the analysis period in the current study.
What are the implications?
The authors conclude that while it is vital to understand the humoral immune response over the long term, some of the commercially available serology assays are not suitable for this use and may account for contradictory research results from convalescent patients. However, males are found to produce more antibodies after infection, though these also fall more steeply.
Many earlier studies show that women have a more robust immune system and also have a markedly higher incidence of autoimmune disease. The stronger T cell response also affects the duration of the immune response in women. This has been attributed to sex hormones, and X chromosomes. The question remains: Would vaccination produce a longer duration of protection in women in view of these findings?
Future directions
The study concludes, “Future work will help in determining whether the sex differences reported here are amplified over longer periods of time, and may be linked to differences in antigen persistence.”
Other subgroups, such as the asymptomatic majority, those with very severe illness and volunteers who have received vaccines during trials, should also be studied in more detail.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources