Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a newly-emergent betacoronavirus, resulted in the COVID-19 pandemic, infecting over 56.75 million people and claiming over 1.35 million lives worldwide to date.
Two other zoonotic betacoronaviruses, SARS-CoV and MERS-CoV, also resulted in outbreaks over the last 20 years. All three viruses are thought to have originated in bats, with SARS-CoV and MERS-CoV adapting to intermediary animal hosts before jumping to humans.
SARS-like viruses circulate in bats, and serological surveillance of people living near caves where bats carry diverse coronaviruses demonstrated direct transmission of SARS-like viruses with pandemic potential, suggesting a pan-coronavirus vaccine is needed to protect against future outbreaks and pandemics. In particular, the WIV1 and SHC014 bat strains are thought to represent an ongoing threat to humans.
In this context, Pamela J. Bjorkman and colleagues have designed nanoparticles co-displaying the SARS-CoV-2 RBD (receptor binding domain) along with RBDs from animal beta coronaviruses that represent threats to humans (mosaic nanoparticles; 4-8 distinct RBDs).
They also made nanoparticles displaying the receptor-binding domain (RBD) of only SARS-CoV-2 (homotypic nanoparticles) and compared the cross-reactivity between the two and the antibodies from COVID-19 convalescent human plasmas.
The team from the California Institute of Technology, and The Rockefeller University, observed that mice immunized with RBD-nanoparticles elicited cross-reactive antibody binding and neutralization responses, confirming increased immunogenicity from multimerization. In contrast, they did not observe this with soluble antigen.
Vaccine candidates against SARS-CoV-2 include the spike trimer protein (S), and the receptor-binding domains (RBDs; S1B domains). Because of its role in viral entry, the spike protein is the primary target of neutralizing antibodies, with many targeting the RBD.
Multivalent display of antigen enhances the B-cell responses. It provides longer-lasting immunity than monovalent antigens. The researchers have designed a coupling strategy using the SpyCatcher-SpyTag system to prepare a multimerized SARS-CoV-2 RBD or S trimer. It elicits high titers of neutralizing antibodies. For designing the mosaic nanoparticles, they prepared the SpyCatcher003-mi3 nanoparticles, simultaneously displaying SpyTagged RBDs from human and animal coronaviruses; to evaluate whether mosaic particles generate cross-reactive antibody responses.
In this study, published on the preprint server bioRxiv*, the researchers immunized mice with the adjuvant plus either soluble SARS-CoV-2 spike trimer (SARS-2 S), nanoparticles displaying only SARS-2 RBD (homotypic SARS-2), nanoparticles co-displaying RBDs (mosaic-4a, mosaic-4b, mosaic-8), or unconjugated nanoparticles (mi3). The three types of mosaic nanoparticles used in this study are mosaic-4a (coupled to SARS-2, RaTG13, SHC014, and Rs4081 RBDs), mosaic-4b (coupled to pang17, RmYN02, RF1, and WIV1 RBDs), and mosaic-8 (coupled to all eight RBDs). These were compared with the homotypic mi3 particles constructed from SARS-CoV-2 RBD alone (homotypic SARS2).
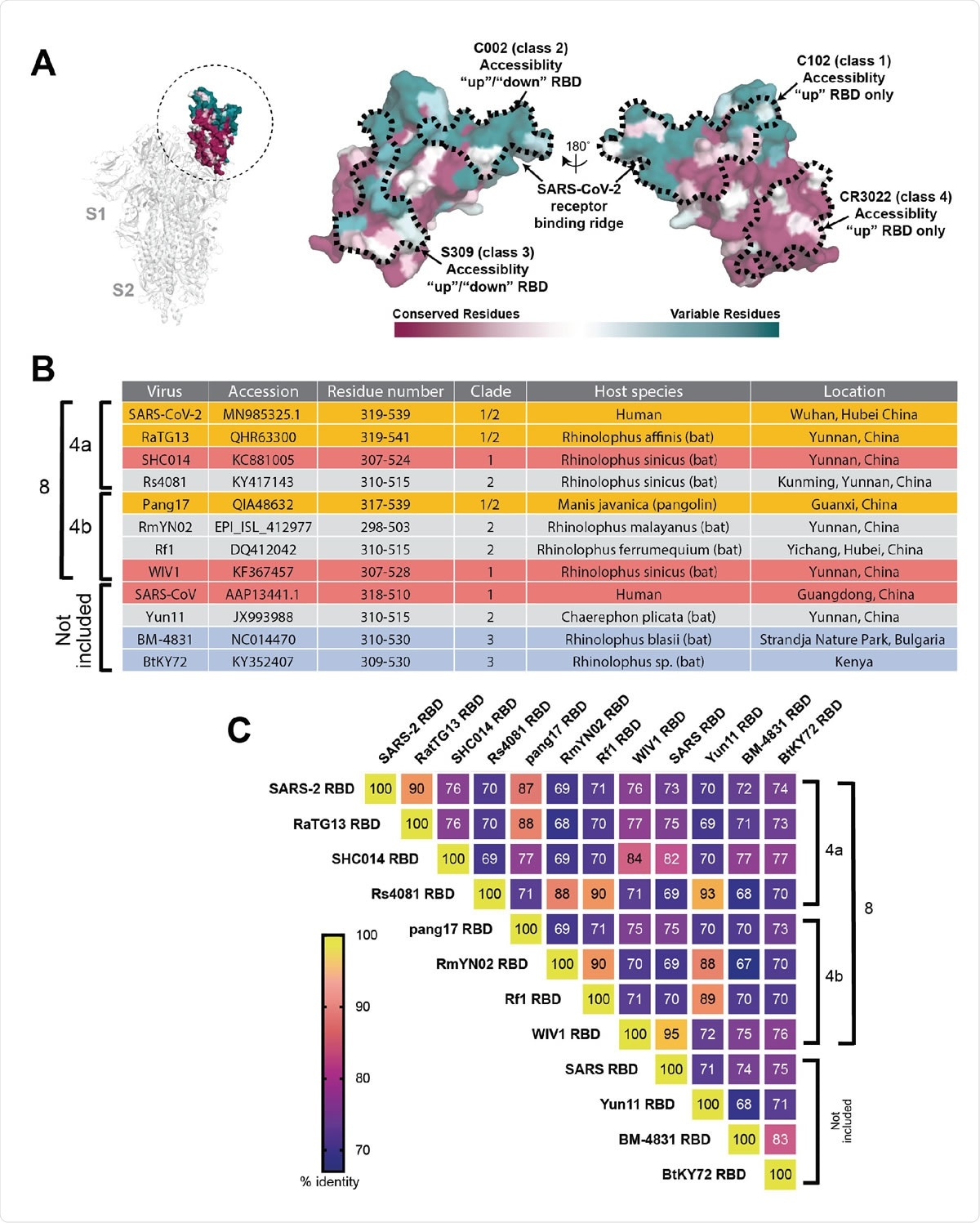
Properties of RBDs chosen for this study. (A) Left: Structure of SARS-CoV-2 S trimer (PDB 6VXX) with one RBD (dashed circle) in an “up” position. Middle and right: Sequence conservation of 12 RBDs calculated by the ConSurf Database (49) plotted on a surface representation of the RBD structure (PDB 7BZ5). (B) Summary of properties of the viral strains from which the 12 S protein RBDs were derived. (C) Heat map showing percent amino acid sequence identities between the 12 RBDs.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The researchers show that the mice immunized with homotypic or mosaic nanoparticles produced broad binding and neutralizing responses, in contrast to plasma antibodies elicited in humans by SARS-CoV-2 infection. The RBD nanoparticles induced cross-reactive IgG responses in the immunized mice.
They found that the mosaicRBD-nanoparticles elicited antibodies with superior cross-reactive recognition of heterologous RBDs compared to sera from immunizations with homotypic SARS-CoV-2–RBD-nanoparticles or antibodies from COVID-19 convalescent human plasmas.
Moreover, the sera from mosaic-RBD– immunized mice neutralized heterologous pseudotyped coronaviruses. The response was equivalent or better after priming compared to the sera from homotypic SARS-CoV-2–RBD-nanoparticle immunizations. This demonstrates that there is no loss of immunogenicity against any particular RBD due to co-display.
The team then investigated the potential for cross-reactive recognition. They questioned whether the B-cell receptors on IgG+ splenic B-cells from RBD-nanoparticle–boosted animals could simultaneously recognize RBDs from SARS-2 and Rs4081 (related by 70% sequence identity). The flow cytometric results showed that the B-cells recognized SARS-2 and Rs4081 RBDs simultaneously. This suggests the existence of antibodies that cross-react with both RBDs.
Also, the researchers compared the antibodies induced by RBD-nanoparticle immunization and the antibodies induced by SARS-CoV-2 infection. They found that the IgGs from convalescent COVID-19 plasma showed little to no cross-reactive responses. This result is consistent with previous studies, where there is little to no cross-reactive recognition of RBDs from zoonotic coronavirus strains resulting from SARS-CoV-2 infection in humans.
In conclusion, the researchers found that the mosaic nanoparticles show enhanced heterologous binding and neutralization properties against human and bat SARS-related coronaviruses compared with homotypic SARS-CoV-2 nanoparticles.
The emergence of the recent viral outbreaks highlights the continued risk of cross-species transmission that may lead to epidemic or pandemic diseases. In this context, the current study provides a vital immunization strategy that may broadly shield us against the coronaviruses. A single immunization with mosaic-RBD-nanoparticles can provide a potential strategy to protect against SARS-CoV-2 and emerging zoonotic coronaviruses simultaneously, the researchers write.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Mosaic RBD nanoparticles elicit neutralizing antibodies against SARS-CoV-2 and zoonotic coronaviruses, Pamela J Bjorkman, Alexander A Cohen, Priyanthi N.P. Gnanapragasam, Yu E. Lee, Susan Ou, Leesa M. Kakutani, Jennifer R Keeffe, Christopher O Barnes, Michel C. Nussenzweig, bioRxiv 2020.11.17.387092; doi: https://doi.org/10.1101/2020.11.17.387092, https://www.biorxiv.org/content/10.1101/2020.11.17.387092v1
- Peer reviewed and published scientific report.
Cohen, Alexander A., Priyanthi N. P. Gnanapragasam, Yu E. Lee, Pauline R. Hoffman, Susan Ou, Leesa M. Kakutani, Jennifer R. Keeffe, et al. 2021. “Mosaic Nanoparticles Elicit Cross-Reactive Immune Responses to Zoonotic Coronaviruses in Mice.” Science 371 (6530): 735–41. https://doi.org/10.1126/science.abf6840. https://www.science.org/doi/10.1126/science.abf6840.