The coronavirus disease 2019 (COVID-19) pandemic continues to spread across the globe. To date, there have been over 59.17 million confirmed infections and over 1.39 million deaths. The causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, China, in December 2019.
Understanding the scale of the virus and its prevalence in a population is an essential part of containing its spread. Therefore, accurate, efficient and affordable testing is crucial in implementing effective social distancing measures, managing local outbreaks and breaking chains of transmission.
But better testing with higher sensitivity can also help predict COVID-19 severity in patients early on in their bout with the virus. Understanding early a patient’s likely prognosis with the disease through high sensitivity testing could allow for necessary therapeutic interventions that would mitigate its potential impact on those vulnerable to more severe or critical cases.
Research laboratories have been working round the clock to develop better detection tests with high specificity and sensitivity. These tests have aimed to detect the neutralizing antibodies (NAbs), which block the receptor-binding domain (RBD) of the spike (S) glycoprotein of SARS-CoV-2.
This NAb-RBD-binding blocks the virus’s protein from interacting with the human angiotensin-converting enzyme 2 (ACE2) receptor, found abundantly in epithelial cells lining the respiratory and intestinal tracts, which is the means by which the virus gains entry into the host cell.
Early humoural immune response to SARS-CoV-2 may predict the clinical outcome (recovery, severity, or mortality). However, due to the lack of highly sensitive serologic testing methods during the key early days of infection, this speculation has not yet been fully investigated.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
In a recent preprint paper released on the medRxiv* server, researchers in New York, USA, have found an association of mortality with an early humoral response to SARS-CoV-2 infection within the first few days after onset of symptoms (DAOS), using a highly sensitive and automated testing-on-a-probe (TOP) biosensor assays for SARS-CoV-2.
The study
The researchers report two sensitive and automated TOP biosensor assays for SARS-CoV-2 viral-specific total antibodies (TAb) and surrogate neutralizing antibodies (SNAb). The competitive binding assay detects the SARS-CoV-2 viral-specific total antibodies (TAb) and surrogate neutralizing antibodies (SNAb), employing an RBD-coated quartz probe.
It also uses a Cy5-Streptavidin polysaccharide conjugate to improve sensitivity and minimize any interference.
The total assay time is 16 min. The researchers also designed the assay with disposable cartridges containing pre-dispensed reagents, meaning it requires no liquid manipulation or fluidics during testing.
The researchers tested the clinical utility of the biosensor by evaluating early antibody responses in real-time polymerase chain reaction reverse transcription (RT-PCR) COVID-19-positive hospitalized patients. The study cohort involved 120 adult patients, who were hospitalized at New York Presbyterian/Weill Cornell Medical Center (NYP/WCMC) from March 8 to April 7, 2020.
COVID-19 severity is observed with different outcomes, leaving doctors, healthcare givers and researchers with no clue to the possible outcome during the treatment or hospitalization of patients. Discrepancies in the observed studies so far depend on a variety of factors.
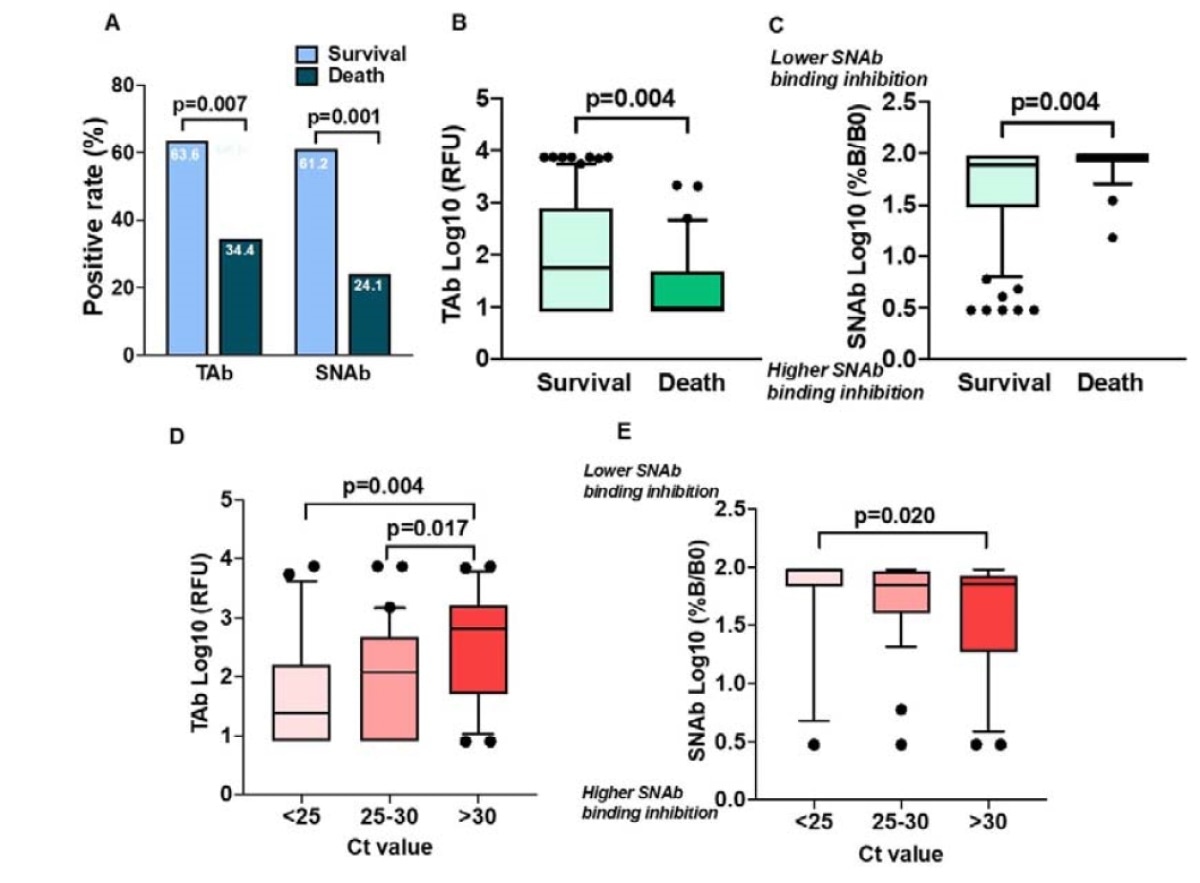
For the first time, the researchers demonstrate in this study that SARS-CoV-2 TAb and SNAb (upon initial presentation) are risk indicators for in-hospital mortality. This study uses the TOP assay to associate the antibodies present on the first day of a hospital visit and subsequent mortality.
Survival probability among SARS-CoV-2 infected patients with positive and negative (A) TOP-TAb and (B) TOP-SNAb at initial hospital ED presentation. Data were analyzed using Cox proportional hazards regression adjusting for age and cancer comorbidity.
The researchers found that the patients who did not present high antibodies during the hospital visit were at a higher risk of in-hospital mortality. Early after infection, the NAbs, if present, avoid severe disease manifestation - by limiting the number of host cells that become productively infected.
They show that the higher antibody levels are associated with a lower viral load.
Their findings agree with recent reports that early immunologic protection in COVID-19 patients is important. These antibodies that appear early in the infection stage can be postulated to protect the patient from the severity of infection and mortality; either by playing a protective role directly or suppressing the SARS-CoV-2 replication indirectly.
A reliable and versatile serological or antibody test detects infection early on. This type of test is still not available on the market.
Conclusion
This study reports a novel, rapid, highly sensitive and fully automated biosensor technology (TOP) that can be adapted to the clinical laboratory setting. The new assays detect early SARS-CoV-2 antibodies on the first day of a hospital visit. The findings show that the levels of SARS-CoV-2 antibodies are inversely associated with the subsequent COVID-19 mortality.
The associations between early antibody response to SARS-CoV-2, initial viral load and eventual in-hospital survival are consistent with strong, early humoral immunity countering SARS-CoV-2 replication - this could have significant implications for managing their immediate and future care.
The researchers believe future studies will explore if these new sensitive and specific assays could potentially monitor the efficacy of antiviral therapies as well as assess antibody responses during vaccine trials.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Yang, He S. et al. (2020) ‘Testing-on-a-probe biosensors reveal association of early SARS-CoV-2 total antibodies and surrogate neutralizing antibodies with mortality in COVID-19 patients.’ medRxiv 2020.11.19.20235044; doi: https://doi.org/10.1101/2020.11.19.20235044, https://www.medrxiv.org/content/10.1101/2020.11.19.20235044v1
- Peer reviewed and published scientific report.
Yang, He S., Sabrina E. Racine-Brzostek, Mohsen Karbaschi, Jim Yee, Alicia Dillard, Peter A.D. Steel, William T. Lee, et al. 2021. “Testing-On-a-Probe Biosensors Reveal Association of Early SARS-CoV-2 Total Antibodies and Surrogate Neutralizing Antibodies with Mortality in COVID-19 Patients.” Biosensors and Bioelectronics 178 (April): 113008. https://doi.org/10.1016/j.bios.2021.113008. https://www.sciencedirect.com/science/article/pii/S0956566321000440.