As it becomes clear that coronavirus disease 2019 (COVID-19) will be around for a long time, there are increasing concerns around the possibility of reinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen that causes COVID-19. The robustness of pre-existing immunity against SARS-CoV-2, due to cross-reactive seasonal coronavirus-targeted immunity, is also of increasing interest among researchers.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The chief viral antigens include the spike or S protein, made up of S1 and S2 subunits, and the nucleocapsid or N protein. The S1 subunit contains the receptor-binding domain (RBD), which binds to the angiotensin-converting enzyme 2 (ACE2) receptor on the host cell membrane. Neutralizing antibodies are expected to work by preventing the binding of the virus via the RBD to ACE2.
A team of scientists in Japan speculated that the severity of COVID-19 in about 20% of infected individuals has something to do with an excessive antibody response. In order to understand this better, they aimed to outline the varying patterns of antibody development in populations in relation to disease severity.
Their current study aimed at delineating the variations in antibody response to N and S1 in a group of around 230 patients with varying severities of COVID-19.
IgM antibodies against N were persistently low in severe and critical disease, peaking at 15 days from the earliest symptom, while IgM against S1 was low in all groups – mild, severe and critical. In the first 10 days, the OD values for IgM-N and IgM-S1 were higher in mild cases relative to critical. With the former, there was then a rapid increase in OD values such that between 11-21 days in mild and severe cases and mild and critical cases, both sets of OD values showed a significant difference. This was absent with IgM-S1 until 22 days, when both sets of values merged.
The peak IgG-N was at around 18 days from symptom onset in severe and critical, thus showing concordance with IgM-N. However, in mild cases, it was delayed to 25 days. The earlier peaks were also the higher ones.
IgG-S1 peaks occurred later, at about 28 days from symptom onset, in both mild and critical cases, but at 40 days in severe illness. The IgG-S1 titers were proportional to disease severity. The OD values between the three groups were significant with both IgGs, but the differences evened out at 22 days.
In severe cases, a strong IgM-N pattern was seen in around 7% and 35% of severe and critical cases, respectively. This was marked by a simultaneous increase in both IgM and IgG antibodies against N. The other patients showed a weak IgM-N response. In both, the OD values of IgG-N were found to peak before IgG-S1.
More patients were found to seroconvert for IgG than for IgM against N and S1. IgG-N antibodies were found in all patients except for one in the mild group. However, IgM-N antibodies were more frequently found in severe and critical cases than in mild cases, at 71%, around 61% and around 30%, respectively. IgM-S1 and IgG-S1 antibodies followed the same trends as IgM-N, the former being found in only ~9% of mild but around 44% each of severe and critical cases. IgG-S1 seroconversion was also high, at around 48% of mild cases, but around 90-94% of severe and critical cases.
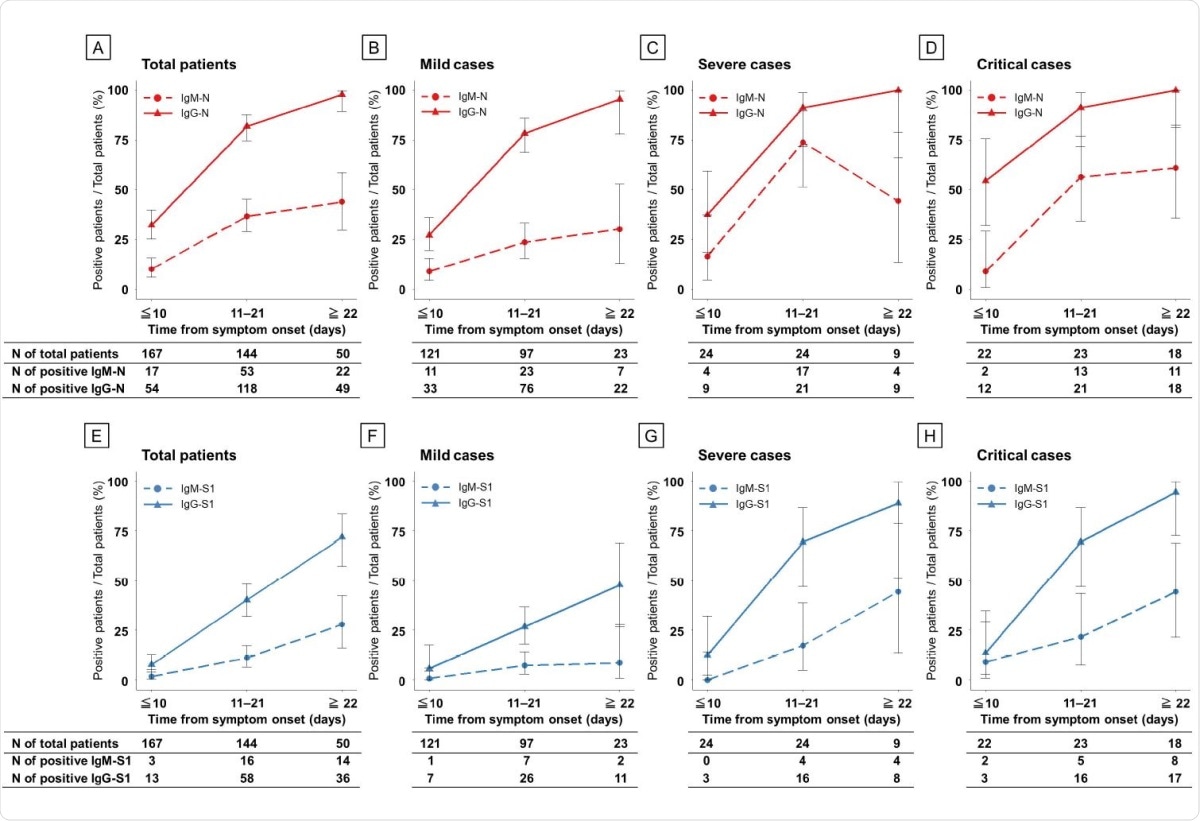
Seroconversion rate of ELISAs in patients with COVID-19 according to disease severity during the clinical course. Antibodies against N protein (A–D) and S1 protein (E–H). Red, N protein; blue, S1 protein; dashed line, IgM antibody assay; and solid line, IgG antibody.
The study shows a simultaneous increase in acute-phase IgM-N and subacute-phase IgG-N, with peak OD values for both being seen at the same time. IgG seroconversion rates were higher throughout the clinical course. This is remarkable because, in many viral infections, the primary exposure leads first to IgM antibodies and then IgG antibodies.
In subsequent exposures to the same virus, IgM and IgG antibodies either appear at the same time, or IgGs appear earlier. Moreover, the IgG titer shoots up after infection, but the IgM titer falls to below that seen during the ‘priming’ infection.
The antibody patterns observed here support previous findings from an earlier study, which attributed this to cross-reactive antibodies to SARS-CoV-2 and other human coronaviruses. This may be the case with the current study, since Japan is not known to have harbored MERS or SARS outbreaks, but seasonal coronavirus epidemics do occur every winter, which should correlate with a high seroprevalence.
In other words, the antibody response is one that corresponds to a secondary immune response to a re-exposure. This could also explain why the seroprevalence for IgM antibodies to SARS-CoV-2 N and S1 is falsely low in Japan, leading to a large number of false-negatives.
The investigators also postulate that the stronger IgM and IgG responses to N and IgG-S1 responses observed in severe and critical cases may contribute to the clinical severity via antibody-dependent enhancement (ADE). This phenomenon is mediated by non-neutralizing antibodies that attach to host cells as well as to the virus, allowing the virus to gain proximity to these cells and to enter and infect them.
This causes a high viral load, which in turn causes an exaggerated cytokine cascade from both the infected cells and from activated T cells, leading to a cytokine storm. This has long been associated with increased severity of COVID-19 and mortality. In support of this, the IgM-N antibodies were increased in only severe and critical cases.
During the Ebola outbreak, ADE was correlated to the presence of IgM antibodies to the virus, and to rapid viral spread within host tissues in early infection.
The findings also agree with earlier research showing that mild COVID-19 is often associated with feeble seroconversion and IgG-S1 or neutralizing antibody production.
Further studies using different techniques will help to confirm these observed antibody patterns, to detect the presence of cross-reactivity to seasonal coronaviruses, and to distinguish between ADE-induced severity of disease and disease-induced high antibody levels.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Imai, Kazuo, Yutaro Kitagawa, Sakiko Tabata, Katsumi Kubota, Mayu Nagura‐Ikeda, Masaru Matsuoka, Kazuyasu Miyoshi, et al. 2021. “Antibody Response Patterns in COVID‐19 Patients with Different Levels of Disease Severity in Japan.” Journal of Medical Virology 93 (5): 3211–18. https://doi.org/10.1002/jmv.26899. https://onlinelibrary.wiley.com/doi/10.1002/jmv.26899.
- Peer reviewed and published scientific report.
Imai, Kazuo, Yutaro Kitagawa, Sakiko Tabata, Katsumi Kubota, Mayu Nagura‐Ikeda, Masaru Matsuoka, Kazuyasu Miyoshi, et al. 2021. “Antibody Response Patterns in COVID‐19 Patients with Different Levels of Disease Severity in Japan.” Journal of Medical Virology 93 (5): 3211–18. https://doi.org/10.1002/jmv.26899. https://onlinelibrary.wiley.com/doi/10.1002/jmv.26899.