We have been working on adrenergic receptors for a few years, in the context of its role in cancer progression and possibly to target them for cancer treatment. Adrenergic receptors are also involved in regulating the immune system and inflammation.
When the initial data on inflammation associated with COVID-19 was published, we realized that the processes involved, namely the activation of inflammatory T lymphocytes and cytokines release, were similar to what we have observed when beta2-adrenergic receptors are stimulated in cancer settings.
.jpg)
Image Credit: Andrii Vodolazhskyi/Shutterstock.com
What are the three stages of SARS-CoV-2 infection?
The progression of COVIDS-19 has been divided into three main stages: stage I—viral response, stage II—pulmonary phase, and stage III—hyper inflammation phase.
Once the patients enter stage III, they will most likely need ventilation and it becomes difficult to manage.
Why is it of high importance that we are able to find an effective therapy to slow down or stop the progression of the virus into its third stage?
The third stage is the last and most severe stage whereby the patients are affected by acute respiratory distress syndrome (ARDS), requiring ventilation in an intensive care unit. This is the stage in which hyper inflammation due to cytokine storms can induce small blood clots throughout the bloodstream, blocking small blood vessels, causing death.
Preventing the progression to this phase could possibly give the needed time for the body to recover as it is not the virus itself that causes the damage, rather the excessive immune response to the virus.
Why are patients with high blood pressure, diabetes, and heart disease at a higher risk of developing a severe infection?
It is a known fact that people with diabetes are at higher risk of severe complications from infection in general, as increased blood sugar levels can reduce the ability of the immune system to fight infection. On the other hand, an acute infection might raise sugar levels and make it difficult to control.
Similarly, about 30% of hospitalized Flu-patients have diabetes, hence diabetes patients are strongly recommended to receive the flu vaccination. COVID-19 associated severe complications are mainly caused by uncontrollable inflammation following a “cytokine storm” which is the release of abundant inflammatory cytokines that affect blood vessels among other structures.
Diabetes patients as well as patients with hypertension are characterized by increased cytokine release and hyperinflammatory state. Also, inflammation will affect the blood by making it thicker putting organs such as the heart, kidney, and lungs under stress.
One still unconfirmed theory also suggests that people with diabetes and high blood pressure have higher ACE2 receptors, the viral receptors used by SARS-CoV-2 to access the cells and generate infection, making them easier to infect.

Image Credit: Lesterman/Shutterstock.com
In your research, you looked at using beta-blockers to potentially treat COVID-19. What are beta-blockers such as propranolol currently used for?
Beta-blockers are a class of medications that work by temporarily stopping or reducing the action of the beta-adrenergic receptors, involved in the body's natural 'fight-or-flight' response.
In return, they reduce stress on certain parts of the body, such as the heart and blood vessels in the brain. They are prevalently used to manage abnormal heart rhythm, hypertension and to protect the heart from recurring myocardial infarction.
Propranolol is a non-selective beta-blocker because it blocks both beta 1 and beta 2 receptors. Propranolol is no longer used to treat heart conditions, but it has been recently repurposed for several pathologies such as cancer, haemangioma, rheumatoid arthritis, and anxiety.
How is the spread of cancer in the lung similar in its inflammatory profile to COVID-19?
Inflammation and oxidative stress predispose to the development of cancer and promote all stages of tumorigenesis. Cancer cells, as well as surrounding stromal and inflammatory cells, engage with each other to form an inflammatory tumor microenvironment (TME).
Clinical studies using non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin have shown that the inhibition of inflammation reduces the incidence and mortality in many cancers. In addition, specific inhibition of cytokines such as interleukin 1β (IL-1β) with canakinumab, significantly reduces the risk of lung cancer development.
In our model of melanoma lung metastasis, cytokines produced such as interleukin-6, interleukin-1β, interferon-γ are very similar to COVID-19 infections.
What current treatments are being trialed to treat COVID-19? What are some of the limitations of these methods so far?
At the moment approximately 360 drugs are in human trials to treat COVID-19, but dexamethasone (a corticosteroid) has been proven to be significantly effective in clinical trials. Anticoagulants (usually low molecular weight heparins) are also used to reduce blood clots in blood vessels.
Corticosteroids are broad anti-inflammatory drugs that depress the entire immune system, so while they are able to mitigate inflammation, they also reduce the ability of the immune system to fight the virus.
An ideal therapy should target only the dangerous components of immune responses that are responsible for the adverse inflammatory reactions caused by specific cytokines, and spare the ability of the immune system to control the virus.
Can you describe how you carried out your research into beta-blockers and their potential effectiveness as a treatment for COVID-19?
Our research is based on our investigations into the beta-2 adrenergic pathway in cancer. Beta-2 adrenergic receptor (ADBR2) is highly expressed in many kinds of cancers and contributes to cancer growth and spread (metastasis). We have shown that targeting ADBR2 can reduce cancer growth and metastasis thus can be used as an adjunct therapy with the current therapy and help in overcoming drug resistance.
ADBR2 is normally expressed on blood vessels, lungs, and cells of the immune system. We believe that blocking ABDR2 can reduce potentially dangerous immune responses without depressing the entire immune system. Many supporting pieces of evidence show that the major symptoms of COVID-19 infection are associated with over-activation of what is called Th17 response and β2-AR signals have been described to have a central role in promoting Th17 response in disease such as rheumatoid arthritis.
Non-selective beta-blockers have been used in clinical settings to reduce inflammation and Th17 response. So, we believe that a similar effect can be obtained in COVID-19 patients.
Moreover, beta-adrenergic receptor antagonists have been shown to be effective in dissolving blood clots. For these reasons, we suggest that beta-blockers such as propranolol should be considered for clinical trials to treat COVID-19.
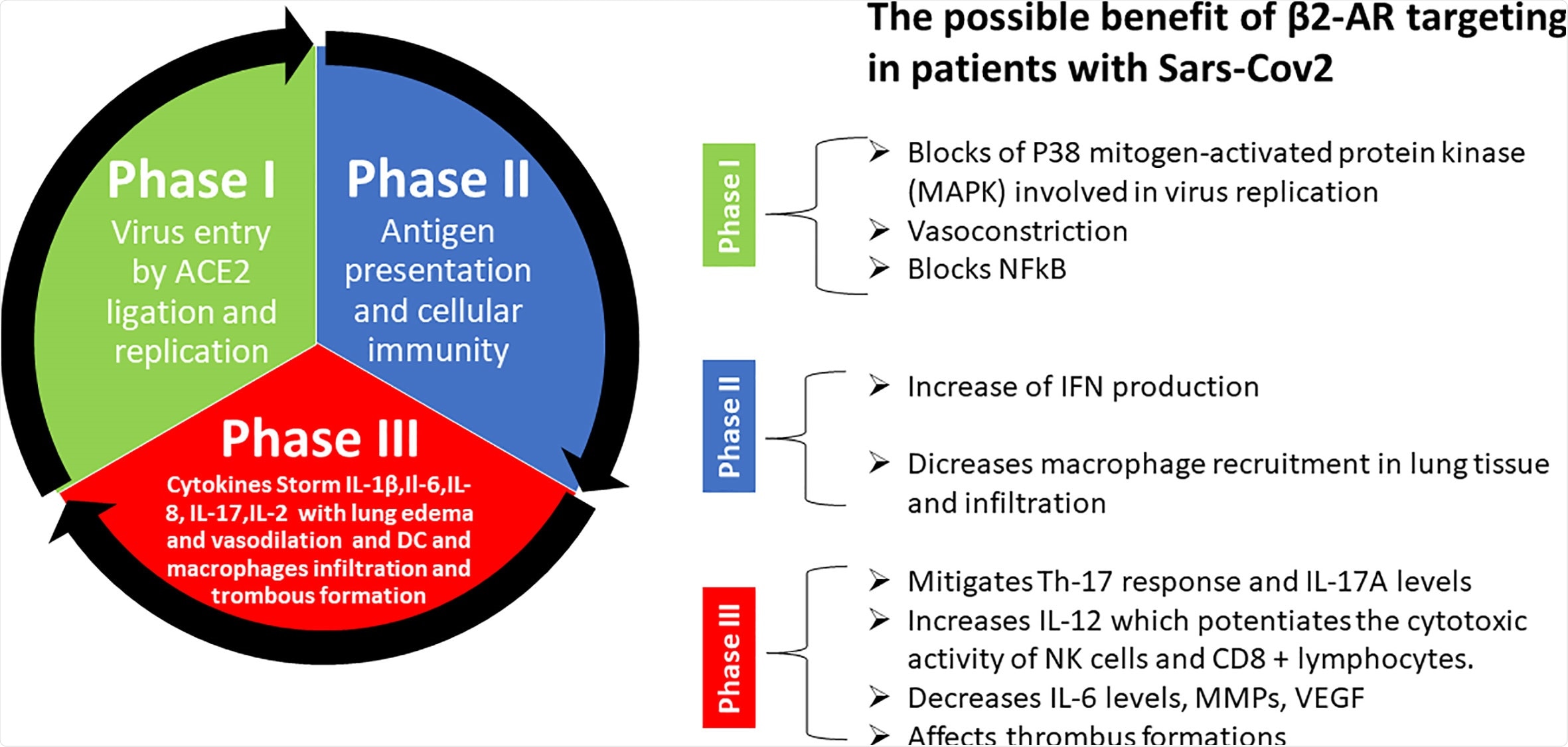
Image Credit: Can Beta-2-Adrenergic Pathway Be a New Target to Combat SARS-CoV-2 Hyperinflammatory Syndrome?—Lessons Learned From Cancer
What did you discover?
We have highlighted the similarity between cancer lung metastases microenvironment and COVID-19 in terms of inflammation and cytokine profile.
We have also pointed out that targeting the adrenergic pathway might be an effective way to reduce inflammation and prevent cytokine storms, thus reducing the risk for patients to enter the most severe phase 3 of the disease.
We strongly believe that this strategy is worth pursuing in a clinical setting.
Can you describe the mechanism behind beta-blockers that help to reduce inflammation and rebalance our immune system?
β2-adrenergic receptors are expressed by all the cells of the immune system, including T and B lymphocytes, dendritic cells (DCs), and macrophages. The specific role of adrenergic signaling in regulating immune responses and inflammation is still under debate.
However, evidence support that activation of β2-adrenergic receptors leads to the generation of reactive oxygen species (ROS) which trigger the secretion of inflammatory cytokines. Moreover, catecholamines (activators of β2-adrenergic receptors) can promote the development of the inflammatory lymphocytes (specifically the Th17 response) which has been recognized to be responsible for the severe immune reaction associated with COVID -19.
In cancer patients, propranolol reduces this inflammatory response, as well as the disease-associated anxiety. Another cytokine IFN- γ can exert direct antiviral effects on infected cells as well as neighboring cells.
Interestingly, propranolol does not lower the levels of this anti-viral cytokine. Therefore, we think that beta-blockers could balance the immune system against SARS-CoV2 by reducing the inflammation that is lethal to patients but at the same time preserve the cytokines which are beneficial to kill the virus.
Do you believe that your research could potentially help to cure COVID-19?
Beta-blockers do not prevent virus replication and spread. On the other hand, the strategy we propose could help in mitigating the cytokine storm induced inflammation in COVID-19 patients thus reducing the lethality.
Image Credit: Shidlovski/Shutterstock.com
What are the next steps in your research into beta-blockers as a potential treatment for COVID-19?
Beta-blockers such as propranolol has been used for decades and are very safe drugs, therefore we believe that it is worthy to conduct a clinical trial on COVID-19 patients at the early stage of the disease to prove its effectiveness.
Where can readers find more information?
- Barbieri A, Robinson N, Palma G, Maurea N, Desiderio V, Botti G. Can Beta-2-Adrenergic Pathway Be a New Target to Combat SARS-CoV-2 Hyperinflammatory Syndrome?-Lessons Learned From Cancer. Front Immunol. 2020 Sep 30;11:588724. doi: 10.3389/fimmu.2020.588724. PMID: 33117402; PMCID: PMC7561388. https://www.frontiersin.org/articles/10.3389/fimmu.2020.588724/full
About Dr. Nirmal Robinson
Nirmal Robinson is the head of Cellular Stress and Immune Response Laboratory at the Centre for Cancer Biology, University of South Australia, Adelaide. Prior to joining the Centre for Cancer Biology, he was a principal investigator at the CECAD Research Centre, University of Cologne, Germany.
Nirmal did his postdoctoral training at the National Research Council of Canada and he holds a Ph.D. from the University of Bonn, Germany. He also holds a master’s degree in pharmacy. His areas of research interests are 1) Mechanisms of cellular adaptation in cancer and bacterial infection, 2) Molecular interface between metabolism and immunity, and 3) Inflammation and Cell death mechanisms associated with cancer and bacterial infections.
About Dr. Vincenzo Desiderio
Vincenzo Desiderio is an associate Professor of Histology and Embryology at the Departments of Experimental Medicine at the University of Campania “Luigi Vanvitelli” of Naples, Italy. He has a master's degree in Pharmaceutical Biotechnology and a Ph.D. in Biotechnology.
His research regards tumor microenvironment, cancer stem cells, and the relation between stem cells and cancer cells. He has authored about 50 publications in peer-reviewed journals and international books.
In 2012 he was awarded the “New Investigator Award” for the I° Symposium on Head and NeckCancer Stem Cells. University of Michigan, Ann Arbor, MI, USA and in 2016 the “Best Researcher under 40” awarded by the Italian Society of Anatomy and Histology.
About Dr. Antonio Barbieri
Dr. Antonio Barbieri is a Researcher and Biologist of IRCSS Istituto Nazionale Tumori Pascale Foundation of Naples Italy.
He works in the Animal Facility Unit under the Scientific Direction of Professor Gerardo Botti and is responsible for animal welfare. He is the Principal Investigator of a project entitled “ Evaluation of the role of Beta2 Adrenergic Receptor (ADRB2) in progression and drug resistance of head and neck and breast cancer”. This project is funded by the Ministry of Health.
To date, He is the author of 76 International papers on cancer and is a Specialist in Clinical Biochemistry.