A recent study published in the journal Phytotherapy Research reveals that resveratrol, a phenolic compound produced by spermatophytes, can inhibit the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative pathogen of coronavirus disease 2019 (COVID-19), in in vitro setups.
As of November 26, 2020, more than 61.1 million confirmed cases of COVID-19, and more than 1.41 million deaths, have been reported globally by the World Health Organization. Although milder COVID-19 cases mostly remain asymptomatic, a severe SARS-CoV-2 infection can give rise to serious complications, such as pneumonia, bilateral lung infiltration, multiorgan failure, and death.
Because of the unavailability of specific therapeutics, in-hospital treatment of severe COVID-19 patients mostly rely on repurposed antiviral drugs, such as remdesivir, ribavirin, penciclovir, lopinavir, and hydroxychloroquine. However, the majority of these drugs are not as effective as expected in treating severe COVID-19 cases. In particular, these drugs are not fully effective in reducing the mortality rate. Given the ever-growing trajectory of COVID-19, it is of prime importance to identify or develop new therapeutics with potent antiviral activity against SARS-CoV-2.
Current study design
The scientists from Shenzhen Third People's Hospital, China, conducted the study to investigate how resveratrol, a phenolic plant compound, can modulate the replication of SARS-CoV-2 in Vero cells. They chose resveratrol as an experimental compound because of its proven efficacy in inhibiting replication of several viruses, including dengue virus, Zika virus, influenza virus, and more importantly, Middle East respiratory syndrome coronavirus (MERS-CoV), which is a betacoronavirus in the same family as SARS-CoV-2.
Firstly, the scientists checked the cytotoxic effect of resveratrol in Vero cells. They observed that even at a very high concentration, resveratrol did not affect the cell viability, indicating that the compound is not harmful to the cells.
They used quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) and immunofluorescence assay to check the effect of resveratrol on SARS-CoV-2 infected cells. They observed a significant inhibitory effect of resveratrol on viral replication.
To determine which phase of the viral life cycle resveratrol exerts its effects, they conducted two sets of experiments. In one set, they treated the cells with resveratrol before infection; and in another set, resveratrol was administered to SARS-CoV-2 infected cells. Interestingly, the findings revealed that resveratrol, when applied before infection, was capable of inhibiting the viral replication only by 20%; whereas, post-treatment with resveratrol caused a 98% reduction in viral replication. These findings suggest that resveratrol is more efficacious in the presence of the virus.
To further investigate the mode of action of resveratrol, they incubated the cells with both SARS-CoV-2 and resveratrol at the same time and observed that resveratrol could inhibit viral replication by 64%. These findings suggest that resveratrol may be capable of preventing viral entry into the cells.
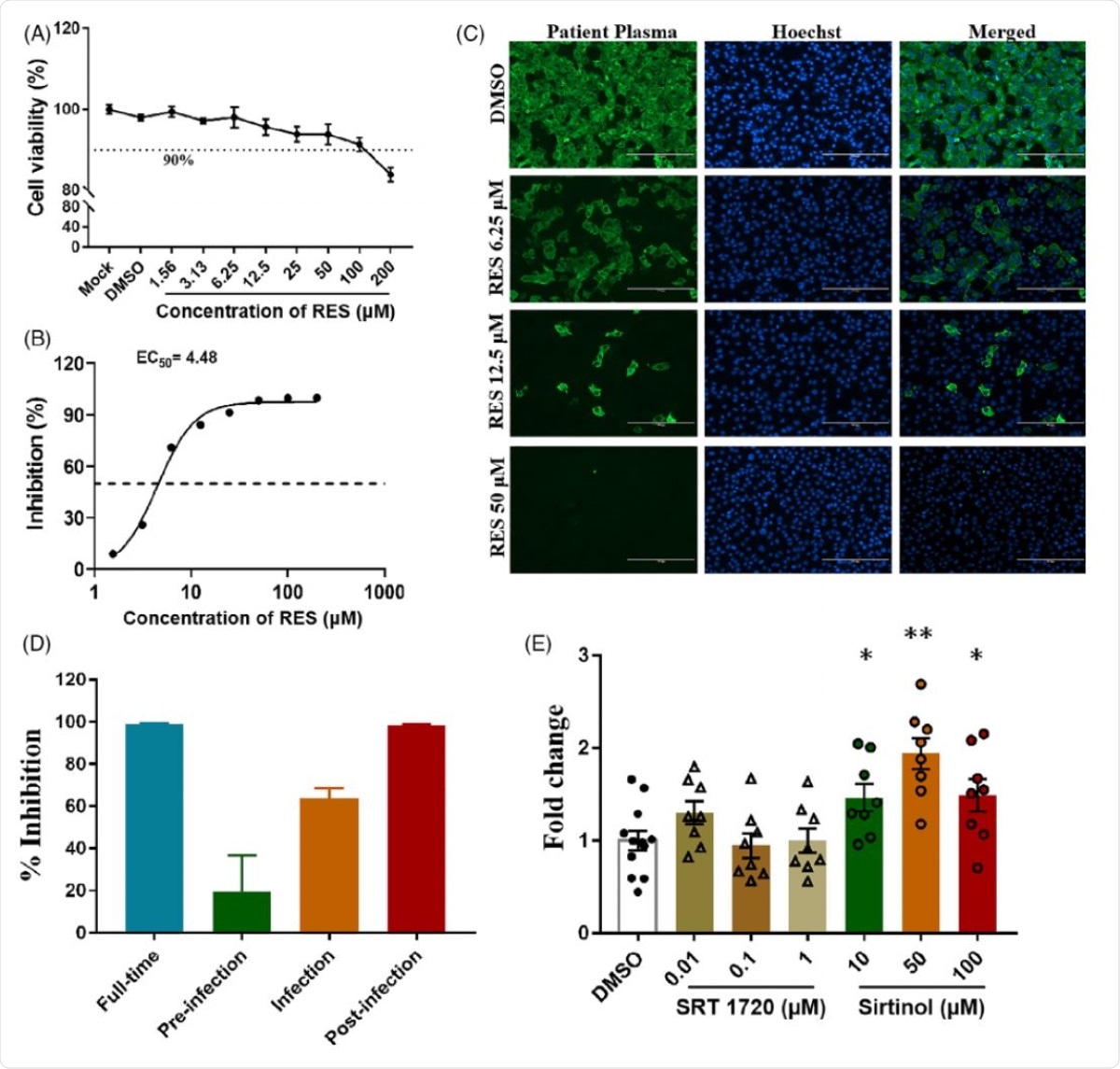
Effects of Resveratrol on SARS-CoV-2 replication in Vero cell culture. (a) Dose-responsive curve of RES on cell survival to test its potential cytotoxicity, the concentration of DMSO was 0.05%. (b) The EC50 of the RES on SARS-CoV-2 in vitro. (c) The representative images of immunofluorescence assay. After 48 hr incubation with virus and RES, cells were fixed, and then probed with COVID-19 patient plasma as the primary antibody, subsequently using Alexa 488-labeled goat anti-humant IgG (1:2,000; Thermo) as the secondary antibody. The nuclei were stained with Hoechst 33342. Scale Bar = 200 µm. (d) Different regimes of RES treatment on the inhibitory rate of SARS-CoV-2 replication. Full-time: Vero cells pre-treated with 50 µM RES for 2 hr, then RES and virus at a multiplicity of infection of 0.01 (the virus titer of this study was 1.08 × 105 TCID50/ml) were simultaneously added into cells for 1 hr. Afterwards, the virus-RES mixture was removed, and the cells were cultured with medium containing 50 µMRES for48 hr.Pre-infectiontreatment: 50 µM RES was added to the cells for 2 hr only, removed and added virus for 1 hr, and then removed the virus and continuously cultured with fresh medium for 48 hr. Co-Infection experiment: RES and virus were simultaneously added into cells for 1 hr, washed and replaced with fresh medium for 48 hr. Post-infection treatment: virus was added to allow attachment for 1 hr, washed and replaced with fresh medium contained 50 µM RES for 48 hr. (e) Effects of SIRT1 antagonist and activator on the inhibitory rate of SARS-CoV-2 replication. After virus infection, drugs with different concentrations were added into cultural medium for 48 hr, Fold change: Normalized to DMSO treatment, “*” represent p < .05, “**” represent p < .01
Given that resveratrol activates SIRT1 signaling, which is important for resisting viral infection, they used a SIRT1 activator (SRT 1720) and an inhibitor (Sirtinol) to investigate whether resveratrol inhibits SARS-CoV-2 replication by modulating SIRT1 signaling pathway. The findings revealed that Sirtinol-mediated inhibition of SIRT1 signaling resulted in induction in viral replication. These findings preliminarily suggest that SIRT1 may be responsible for inhibiting viral replication. According to the scientists, further studies are required to gain more insight into the mechanistic details of this observation.
Study significance
The current study identifies resveratrol as a potential antiviral candidate that is capable of inhibiting SARS-CoV-2 replication. Previously, it has been reported that resveratrol reduces cellular inflammation by decreasing the production of nitric oxide. Moreover, there is much evidence showing that resveratrol acts as a potent antioxidant. Another benefit of resveratrol is that it does not cause any serious adversity even when used at a very high concentration. Because oxidative stress and inflammation are common consequences of any viral infection, the scientists believe that resveratrol holds the potency to be used as an effective therapeutic agent to treat COVID-19 patients.