The onset of colder weather in many parts of the world – particularly the Northern Hemisphere – is predicted to usher in fresh surges in coronavirus disease 2019 (COVID-19) infection rates, hospitalizations and, ultimately, deaths. In order to contain the virus effectively, many models of transmission have been explored.
Transmission 'hot zones' drive COVID-19 pandemic. Image Credit: Optimarc / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Most models of infectious disease follow the SIS or SIR models. The former includes susceptible and infected individuals, and the latter incorporates susceptible, infected and recovered categories. Several investigators have based their studies on the assumption of homogeneous community structure and interactions. Others take account of distinctive patterns based on age groups, spatial destruction, and contact networks. However, COVID-19 shows some unusual patterns that do not fit the conventional infection models.
With respect to COVID-19, the virus is thought to spread “at large” as people go about their daily lives, as with influenza. Within this normal pattern of interactions, there are special events such as the occurrence of superspreading events, characterized by the spread of the virus from one individual to a large number of people.
Hot zones drive the pandemic
In the current pandemic, viral transmission seems to be efficient in certain settings where many people interact in an indoor setting for long periods, such as in restaurants, nursing homes, industrial plants, prisons and dormitories for workers. These are called transmission “hot zones” and can result in many more infections compared to those occurring outdoors and between members of the community in general.
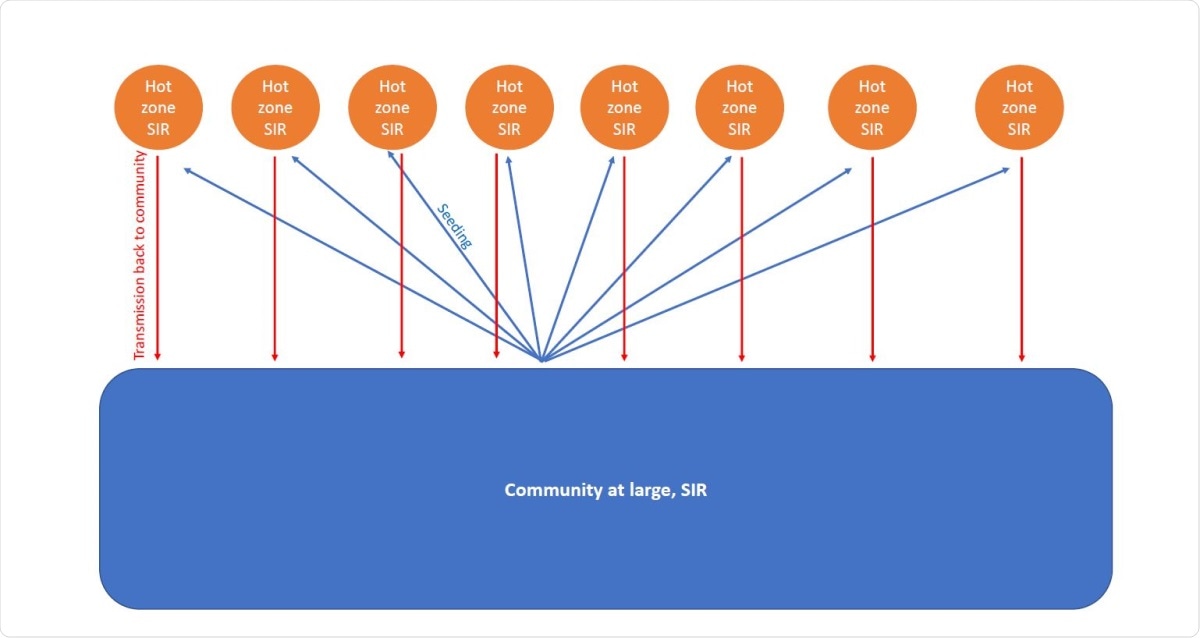
Schematic representation of model assumptions.
Such hot zones may be due to recurrent interactions between the same people, leading to multiplied infectious contacts, causing viral amplification. Earlier research based on these two types of zones showed that efficient spread of infection seems to depend on hot zone spread at high doses. This is despite the undoubted fact that most infections are mild and can occur in the community at large.
The current study focuses on the formation of and spread of the virus within two types of hot zones: static and dynamic.
Static hot zones
The first type is represented by a large compartment, denoting the community at large, and individual hot zones, denoted by patches of small size. Within these zones, including prisons, food processing plants and nursing homes, there are repeated and regular interactions between the same people, leading to a high transmission frequency.
The virus may be brought by someone from the community at large to this hot zone, instigating a wide chain of transmission. Such individuals are likely to be those with mild infection (since severely infected individuals will probably not come to such zones), and to be drawn from a relatively small number of such individuals within the community at large.
The infection then spreads within the hot zone, but its members also return it to the community at large. The higher the prevalence of infection, the greater the percentage of severe infections. Once the prevalence crosses a somewhat high threshold level, all infections are severe.
This again is very close to the pattern seen in COVID-19, where people exposed to a higher dose of the virus become more severely sick. In the US, severe cases were first detected in nursing homes, which again agrees with this model.
The researchers assume a low reproductive rate in the community at large, about 1.25, but higher in the hot zones, at 3.75, because the latter allows a faster rate of spread. Without interventions, this would allow mild infections to spread, seeding hot zones.
This leads to a fast increase in the rate of mild infections in the community, driven by, and similar to, that in the hot zones. More severe community infections arise, perhaps marking the onset of community testing as more symptomatic cases emerge. This is in line with the early situation in the US, according to the researchers. A rapid doubling time was first observed, with a progressive slowing later on.
Extinction of infection in some hot zones is followed by seeding in others. The number of community infections therefore peaks and then drops, so that the R-value declines slowly. This allows the seeding of more hot zones and retrograde community spread. Thus, infections fluctuate up and down around a steady level.
The role of interventions
The study also explores the effects of NPIs, such that without any intervention, the infection would first peak, seeding one hot zone after another. Each hot zone shows an increasing spread of infection, followed by a fall in prevalence towards extinction, at which point the next hot zone is seeded. This process ends when community infection is extinct.
With very early NPIs, infectivity in the community reduces rapidly, once the total percentage falls below 0.01%, and enters a plateau, as in California, where no distinct peak was observed. Hot zones are seeded from the general population, and thus they can harbor the virus. Each hot zone has only a temporary viral presence followed by extinction, but additional hot zones are created over time. This balance keeps the infection rates fluctuating at a more or less steady level until all hot zones are finally infected; the stronger the NPI, the lower the steady level.
If the intervention is delayed until a community infection level of 1%, the infection rate declines more steeply but plateaus thereafter. This resembles the New York State picture. If the NPIs are implemented only at a community infection rate of 15%, the dynamics resemble those of a no-intervention scenario, with a fast extinction of the virus. The hot zones therefore fail to be seeded and no plateau phase is observed in the long term.
This pattern is replicated in areas of high R, with the difference being a much higher cumulative infection level peak at 40% to 60%, due to a higher basal transmission rate.
Dynamic hot zones
The second type of hot zone is the dynamic hot zone, which forms spontaneously and then dissolves. These are composed of people from the community at large who come together for a short time and then disperse again. This is represented by gatherings in movie theaters, bars and restaurants. This model results in a different pattern of virus spread that is in keeping with standard spread patterns, but does not explain the observed unique changes in transmission seen in the current pandemic. The spread of the virus is much faster but agrees with standard SIR models, but do not show infection plateaus
Dynamic hot spot spread accounts for higher transmission rates above the average, and should thus be paid special attention. However, they do not predict the unique spread patterns of the current pandemic and cannot cause its long-term plateau. They would also necessitate different NPIs, such as restricting gatherings at such venues.
What are the implications?
Static hot zone transmission can give rise to COVID-19-specific infection patterns, indicating it could be an important driver of these dynamics. The pattern of infection dynamics is thus sub-exponential, infection plateaus are observed, and community spread is driven by hot zone spread.
The actual community R-value of 1.25 is estimated at 2-3 due solely to hot spot transmission. Without intervention, the infection peak is at 20% of the population, at the lower R-value. This would be very unlikely at the higher R-value. More research is thus required in this area.
These hot zones serve as infection reservoirs that sustain infection at high levels after natural spread or after non-pharmaceutical interventions (NPIs) are put in place. The presence of static hot spot transmission can predict the kind of epidemiological patterns that have been reported in many studies, such as prolonged infection plateaus and sub-exponential growth. Moreover, this also shows the difficulties of estimating the basic reproduction number using community-level transmission data.
With specific hot zone interventions, the R-value there drops below 1, and the infection becomes extinct. Community spread continues until about a fifth are infected and then drops. If hot zone interventions stop before extinction occurs, on the other hand, community transmission continues at the same rate, and more hot zones are seeded, leading to a steady state.
Conversely, the authors point out, “if R0 in the community is lower than that in the hot zones, continued interventions in the hot zones, after interventions have been stopped in the community at large, could help reduce the extent of subsequent infection waves.”
The study concludes, “This classification of infection hot zones into two groups deserves further investigation, especially in the context of determining what types of hot zones should be targeted by interventions to achieve maximal virus suppression.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources