In the race to successfully vaccinate the global population against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, not one but two RNA-based vaccines have completed their final phase of clinical trials and are now awaiting regulatory body approval. In the United States, the institution which grants this final seal of approval is the Food and Drug Administration (FDA); once approved, mass distribution and administration of a vaccine can commence.
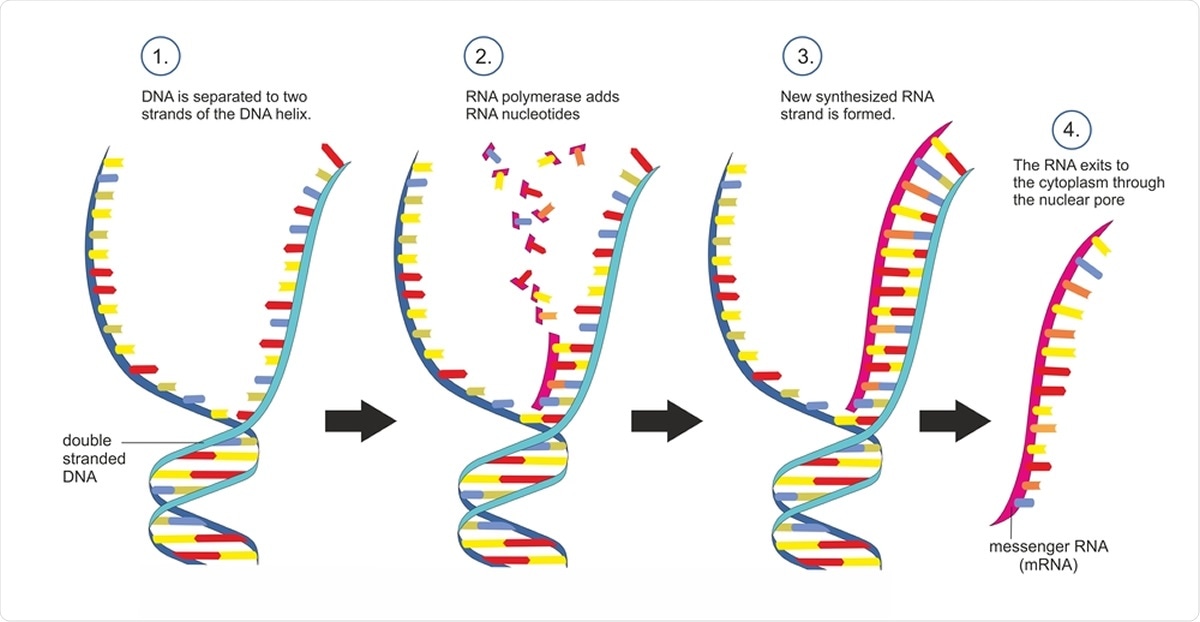
Double-stranded DNA is copied into single-stranded messenger RNA in a transcription. Image Credit: Soleil Nordic
The process of approval for vaccine candidates
Once vaccines are designed, they undergo several stages of rigorous testing and approval before they can enter the market: initially, they 1) enter preclinical testing, conducted on cell cultures and in animals; then 2) they enter phase 1 of clinical trials, conducted on a small sample of human volunteers; then 3) human-based trials are expanded in phase 2, often involving hundreds of participants and including children and the elderly; before 4) the phase 3 trials commence which aim to discern the immunogenic efficacy and safety of the vaccine, usually involving tens of thousands of volunteers; finally, the researchers 5) present the findings from their clinical trials to public health bodies (like the FDA) who scrutinize the data and make a judgment on whether or not the vaccine is sufficiently efficacious and safe for widescale distribution.
The mRNA candidates
In November 2020, two biotech giants — Pfizer and Moderna — came out with potential coronavirus disease 2019 (COVID-19) vaccine candidates, announcing promising outcomes of their respective clinical trials. These candidates — both being the first of a new class of messenger RNA (mRNA)-based vaccines — may become the first effective prophylactic pharmacological measures against SARS-CoV-2 infection.
BNT162b2 is the vaccine developed in collaboration with the small German start-up BioNtech (Biopharmaceutical New Technologies) and the large American pharma corporation, Pfizer. The results from their clinical trials demonstrate 95% efficacy against SARS-CoV-2 infection. It also meets all primary efficacy points, consistent across age, gender, race, and ethnicity demographics.
The other vaccine, mRNA-1273, was developed by the Cambridge, MA-based biotech company Moderna, Inc., in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH). This vaccine demonstrated efficacy of 94.5%.
These two vaccine candidates are made from the mRNA, which contains the message for building the spike protein, essential for the coronavirus’ entry into the host cell. Both BNT162b2 and mRNA-1273 encode for genetic variants of the SARS-CoV-2 spike protein; it is more stable and immunogenic than the natural protein. They enter the host cell and cause them to make these spike proteins. Once endocytosed, and translated into antigenic proteins, the immune system machinery kick-starts, producing neutralizing antibodies that can target SARS-CoV-2’s spike protein if a person is exposed to the pathogen.
For the safe delivery of mRNA, a perfect vector is required. The mRNA cargo must arrive into the cytosol across the cellular plasma membrane without being degraded in the circulation. Lipid nanoparticles are one of the best choices for the mRNA carrier. Within these positively-charged lipids, the mRNAs are safe against RNase-mediated degradation. In the form of self-assembled virus-sized particles, it also becomes easy to administer via different routes.
An mRNA-based therapy has enormous advantages: 1) delivery is safe as it is not infectious compared to a live virus, and it does not integrate into the host genome like a DNA-based delivery; 2) mRNA processing occurs in the cytosol – and so it does not need to enter the nucleus; 3) mRNA has a short half-life, which can be regulated by molecular design; and 4) it is immunogenic (in that it can effectively elicit the robust immune response required in the host to engender immunity). Also, its immunogenicity can be modulated using molecular engineering techniques.
A significant challenge here is a logistical hurdle: mRNA is unstable unless kept in a deep freeze. For transportation, storage, and local delivery - a minus 70 degrees Celsius (minus 94 degrees Fahrenheit) chilled storage is required until it is administered.
A significant milestone for nanomedicine
These vaccines are a groundbreaking innovation in nanomedicine and a tremendous scientific feat realized in an incredibly short period of time. Vaccines typically take years of research before beginning clinical testing.
To date, therapeutic approaches involving nanomedicine have not translated well from ‘lab-to-bedside’. Especially for cancer therapies, the results have been underwhelming. This is attributed to the poor understanding of the complex nano-bio interactions happening.
However, in the case of vaccines, the simple, versatile, and scalable nano-platforms accrue to the success of the nanomedicine approach. The possibility to engineer using standard laboratory techniques means that they can be easily and quickly adapted to produce new vaccines against future epidemics.
COVID-19 has created an unprecedented global health crisis. These nanomedicine-based vaccines may help mitigate the current pandemic and its painful ramifications on the global economy and public health.
The success of the vaccine also depends on the execution of administration by the local governing bodies. The second dosage is imperative for an effective COVID-19 vaccine program. People must be made aware of the importance of both the dosages and comply.
These two nanoparticle-based vaccines are close to approval by the US Food and Drug Administration. However, many questions remain about these vaccines and their outcomes in the real-world setting. If successful, however, they would help to mitigate a global health crisis of unprecedented dimensions in modern history, demonstrating an impactful application of nanomedicine at a global scale and raising awareness about its potential benefits to the broadest audience.