Novel Coronavirus SARS-CoV-2. This scanning electron microscope image shows SARS-CoV-2 (orange)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (green) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Image Credit: NIAID / Flickr
One of the efforts to combat the ongoing coronavirus disease 2019 (COVID-19), pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is repurposing drugs already in use.
Polyether ionophores are a family of natural products used as antibiotics. Often used in animals, they can inhibit gram-positive bacteria, including drug-resistant strains. They have also been seen to have antiviral activity against RNA and DNA viruses like influenza, HIV, and Zika. Studies reported that the ionophores salinomycin and monensin could inhibit Middle East respiratory syndrome (MERS-CoV), a coronavirus pathogen closely related to SARS-CoV-2. While the exact mechanism of antiviral activity in ionophores is unknown, it is likely that they interfere with the virus replication cycle.
Polyether ionophores inhibit SARS-CoV-2
In a recent study published in the journal Antiviral Research, researchers from Aarhus University, Denmark, report on their tests on different polyether ionophores to inhibit SARS-CoV-2 in vitro.
The team tested 11 natural polyether ionophores and one synthetic analog to determine if in vitro changes in Vero E6 cells – which overexpress the human serine protease TMPRSS2 that facilitates viral entry – infected with SARS-CoV-2 virus could be rescued. The virus infection causes highly fluorescent cells, which allowed the authors to image and quantify the changes. As controls for their test, the team used Remdesivir and hydroxychloroquine (HCQ) – two antiviral drugs that have recently been repurposed to treat severe COVID-19 with varying success.
After infecting Vero E6 cells with SARS-CoV-2, the team added the different ionophores and left them for 72 hours. Viral replication was tested using a SARS-CoV-2 TCID50% assay, and Western blot analysis was used to test the cells after lysis.
The authors found that all the 11 ionophores showed some viral inhibition, although with different potencies. Some compounds, including narasin, salinomycin, and nanchangmycin, had more than 100-fold selectivity to SARS-CoV-2. Other compounds, nigericin, indanomycin, monensin, and lasalocid, showed selectivity of about 50–100 fold. The Ca-ionophores ionomycin and calcimycin showed low selectivity of 5- and 18-fold, respectively.
In contrast, the synthetic ionophore HL-201 was not selective and did not inhibit viral replication. Remdesivir showed antiviral activity and had a selectivity of more than 67-fold, but HCQ did not show any antiviral effect.
The compounds maduramycin and X-206 showed were highly selective, 313- and 586-fold selectivity, and highly potent. Using qRT-PCR to test for viral RNA and formation of SARS-CoV-2 spike protein, the authors found X-206 significantly inhibited viral replication, even at a low concentration of 760 pM. Standard plaque assay showed that X-206 greatly reduced the release of virus particles. Furthermore, X-206 also inhibited the replication of the virus in human lung cells.
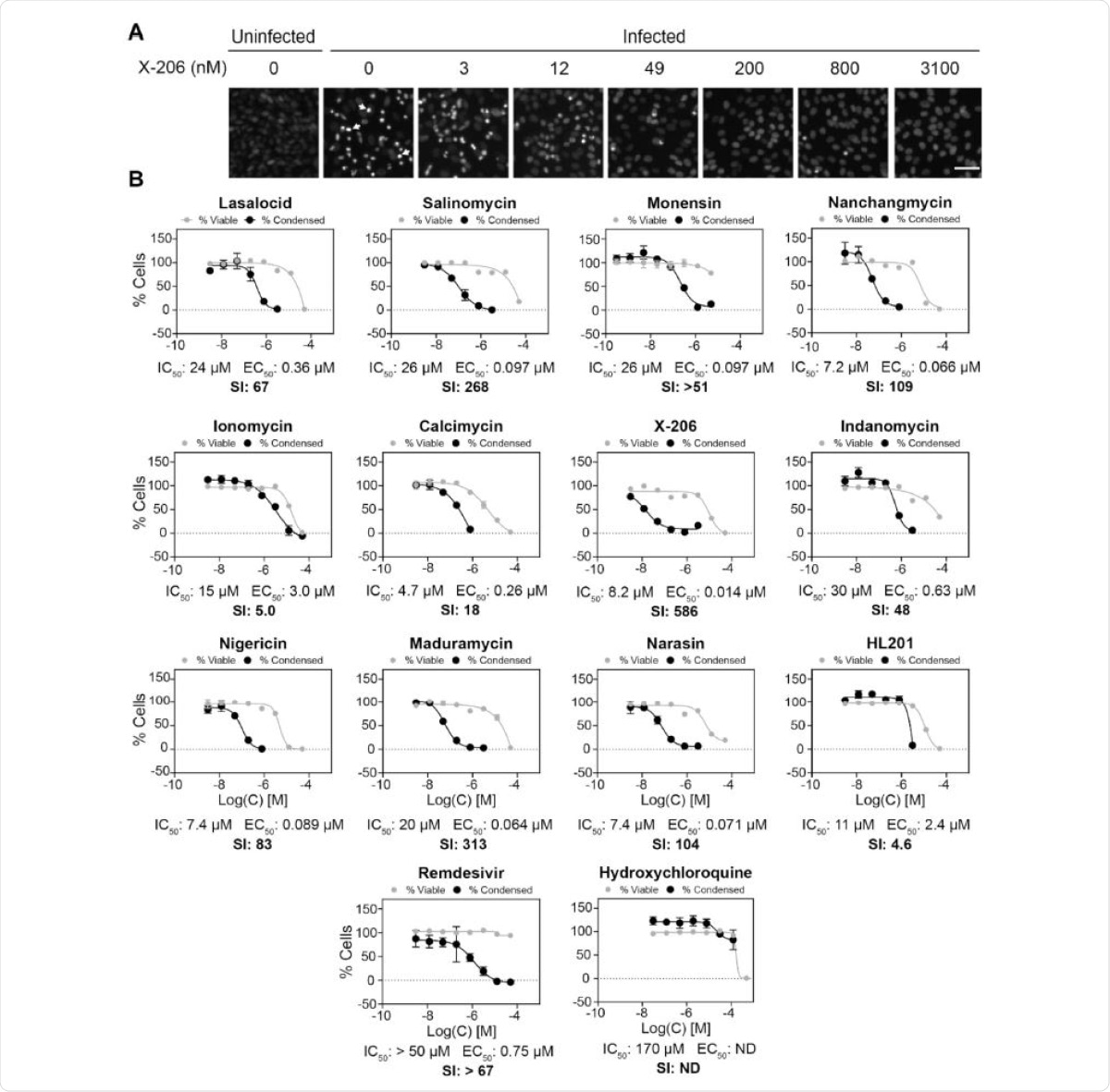
Screen of polyether ionophores for antiviral activity against SARS-CoV-2 infection in Vero E6-hTMPRSS2 cells. (A) Representative microscope images. White arrows show examples of viral-induced condensed cells. Scale bar =50 µm. (B) The relative number of viral-induced condensed cells (black lines) were counted and effects on cell viability of uninfected Vero E6-hTMPRSS2 cells were determined by CellTiter-Blue (grey lines). Data points are mean ±s.d (N =3). SI =selec-tivity index. E.B. Svenningsen et al.
More tests needed to understand potential use against COVID-19
Cationic amphiphiles like HCQ increase cell lysosome pH and disrupt autophagy processes, which may partly be the mechanism by which they inhibit viral replication in vitro. Polyether ionophores are also known to accumulate in lysosomes, affect autophagy, and can change lysosomal pH. Thus, it is likely that polyether ionophores and cationic amphiphiles have similar viral inhibition mechanisms.
Although HCQ was not effective in Vero-E6 expressing TMPRSS2, it effectively inhibited virus replication in wild-type Vero E6 cells. However, the activity of X-206 was independent of TMPRSS2 expression, suggesting a different mechanism of inhibition compared to HCQ.
The authors used morphological profiling in uninfected Vero-E6 expressing TMPRSS2 to understand the mechanism. They found that the bioactivity profiles of HCQ and bafilomycin, a natural product with antiviral activity, are highly correlated, suggesting a similar mechanism, likely lysosomal deacidification.
In contrast, the bioactivity profiles of polyether ionophores were different from HCQ and bafilomycin, but similar to each other. This suggests the antiviral effect of ionophores is different from that of cationic amphiphiles. The bioactive concentrations in the assay were approximately similar to their antiviral concentrations.
The team further analyzed how the antiviral activity of X-206 varied with the timing of addition of the compound after viral infection, up to 8 hours after infection. They found that a 12 nM concentration of X-206 effectively reduced viral infection at 0.5 and 2 hours after infection, including a 2-fold log-reduction of the virus in the supernatant.
The safety and toxicity of polyether ionophores in humans currently unknown. X-206 has been shown to have some toxicity in animals, but some compounds are commercially produced and are used in agriculture.
These findings thus warrant further study to clarify both the antiviral mechanism by which polyether ionophores inhibit viral replication as well as their safety for use against COVID-19 in humans.