Researchers at The Rockefeller University in New York have conducted a study showing that infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19) – triggers persistent T cell responses that last for at least six months.
Gaëlle Breton and colleagues found that individuals who had recovered from COVID-19 exhibited SARS-CoV-2-specific immune memory that could contribute to recall responses on re-exposure to the virus.
Enduring differences in the relative numbers of CD4+ and CD8+ T cell compartments were also observed among recovered individuals, compared with people who had not had COVID-19.
The team says the effect that these alterations have on the overall health of the immune system remains to be determined.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
A pre-print version of the paper is available on the bioRxiv* server, while the article undergoes peer review.
The humoral and cellular responses to SARS-CoV-2 infection
Infection with SARS-CoV-2 elicits both humoral and cellular immune responses. Antibodies target most of the virus’s structural proteins, including the nucleocapsid (NCAP) protein, membrane (Memb) protein, and accessory protein 3a (AP3a). Neutralizing antibodies primarily target the receptor-binding domain of a protein called Spike – the main structure the virus uses to bind to and access host cells.
Cellular immune responses can vary widely, with some studies showing T cell responses that range from undetectable to robust activation of CD8+ T cells and/or CD4+ T cells.
“Although there is increasing evidence that cellular immunity plays a major role in resolution of COVID-19, little is known about the persistence of cellular immunity to SARS-CoV-2,” says Breton and colleagues. “This is a particularly important issue when considering an individual’s ability to resist a second exposure to the virus.”
What did the researchers do?
To investigate whether SARS-CoV-2 infection is associated with enduring cellular immune responses, the researchers examined blood samples collected at 1.3 and 6.1 months post-infection among 41 individuals who had recovered from COVID-19.
Isolated peripheral blood mononuclear cells were stimulated with a collection of pooled SARS-CoV-2 peptides in vitro. The peptides included individual pools of Spike, NCAP, Memb and AP3a. High dimensional flow cytometry was used to compare samples from COVID-19 convalescent individuals with those from people who had not had COVID-19.
At the first timepoint, SARS-CoV-2-antigen-specific CD4+ T cells expressing memory markers and the cytokines interleukin 2 (IL-2), interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) were significantly elevated among the COVID-19 convalescent individuals.
At the second timepoint, the relative frequency of these cells decreased by between 22% and 32%, suggesting that SARS-CoV-2-specific CD4+ T cells are induced during acute infection and are only slightly reduced after 6.1 months.
Further analysis revealed that the IL-2, IFN-γ, and TNF-α responses to the individual pools of Spike, NCAP, Memb, and AP3a were all increased at both time points.
When all cytokines were considered together, a significant increase in antigen-specific CD4+ T cell responses to all of the peptide pools was observed at both time points.
“The increase in CD4+ T cell cytokine responses to the individual peptide pools and the overall combination of all SARS-CoV-2 antigens was not driven by any single cytokine but instead reflected increases in each of the three cytokines measured,” write Breton and team.
What about CD8+ T cell responses?
Unlike CD4+ T cells, CD8+ T cell responses to the peptide pools were much more variable and generally less robust. Although 95% of convalescent samples responded to at least one peptide pool at both timepoints, the proportion of responding cells was low.
However, although fewer-antigen-specific CD8+ T cell responses than CD4+ T cell responses were observed, they still persisted at the 6.1 month timepoint.
Alterations in relative numbers of CD4+ and CD8+ T cells
Recovered individuals also exhibited significant alterations in relative numbers of circulating CD4+ and CD8+ T cell compartments that persisted at 6.1 months.
At 1.3 months, the relative proportions of circulating CD4+ T cells decreased, while circulating CD8+ T cells increased, although both had returned to near physiologic levels by 6.1 months.
Furthermore, expression of programmed cell death protein 1 (PD-1) – a regulator of autoimmunity – was decreased on both CD4+ and CD8+ T cells at both timepoints.
“We conclude that there are persistent changes in the distribution of circulating CD4+ and CD8+ T cells and their expression of activation/exhaustion markers,” writes the team.
The proportion of central memory CD4+ and CD8+ T cells was also decreased at both time points, while the proportion of cycling CD4+ and CD8+ T cells increased at both time points.
Again, PD-1 expression was decreased on central memory and cycling cells at both time points.
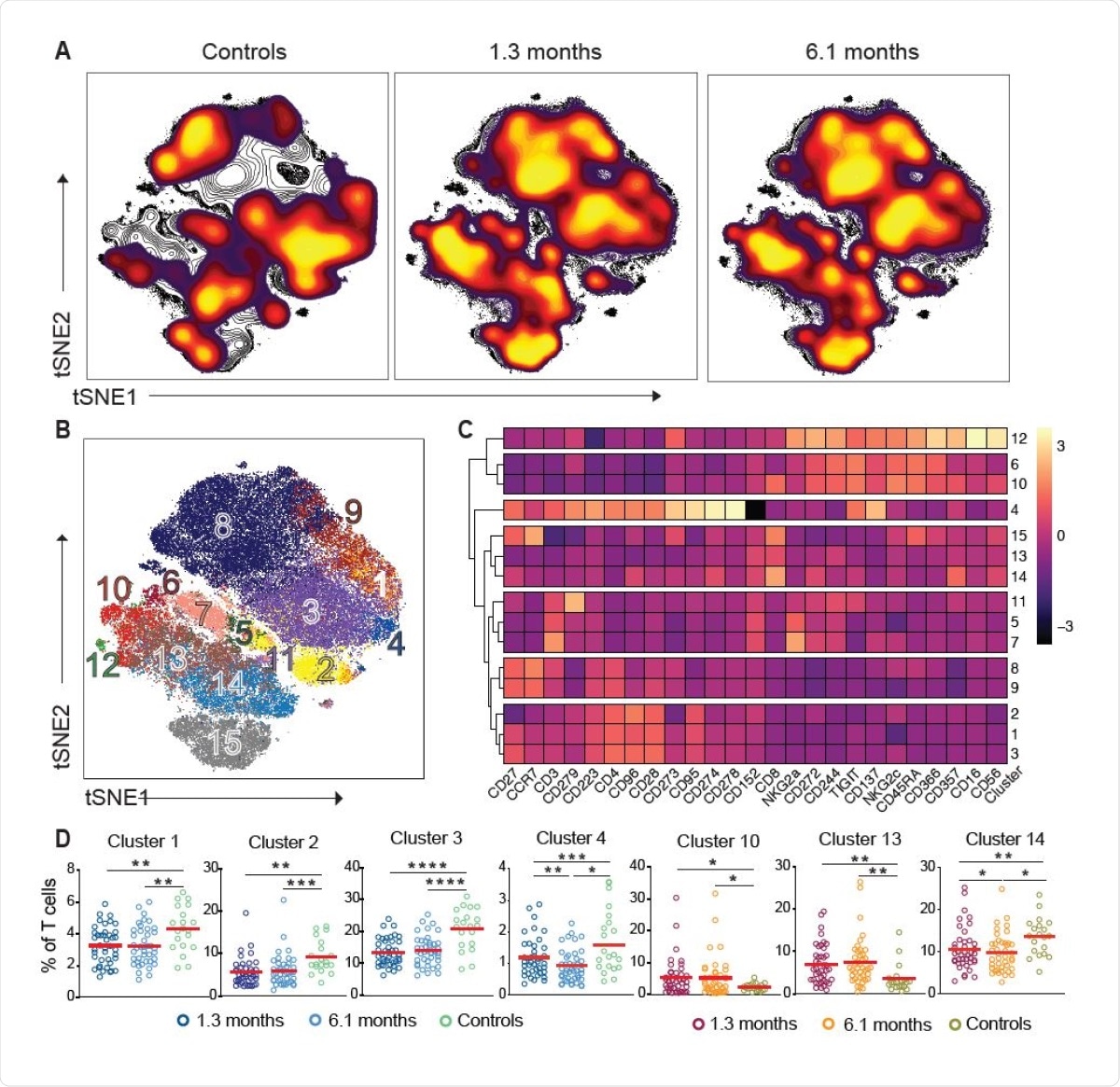
Persistent longitudinal changes in the phenotypic landscape of T cells in individuals recovered from COVID-19. (A) Global viSNE projection of pooled T cells for all participants pooled shown in background contour plots, with overlaid projections of concatenated controls, convalescent patients 1.3 months, and convalescent 6.1 months, respectively. (B) viSNE projection of pooled T cells for all participants of T cell clusters identified by FlowSOM clustering. (C) Column-scaled z-scores of median fluorescence intensity (MFI) as indicated by cluster and marker. (D) Frequency of T cells from each group in FlowSOM clusters indicated. Each dot represents an individual with COVID-19 at 1.3 months (dark blue for CD4+ T cells and dark red for CD8+ T cells) or 6.1 months (light blue for CD4+ T cells and orange for CD8+ T cells) as well as control individuals (green).
The impact of the abnormalities on overall immune health remains to be determined
The researchers say the findings show that CD4+ and CD8+ T cell subset distribution, cell division and expression of activation/exhaustion markers remain altered 6 months after SARS-CoV-2 infection.
“These abnormalities were not directly associated with persistent symptoms and their impact on the overall immune health of the individual remains to be determined,” conclude Breton and team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Breton G, et al. Persistent Cellular Immunity to SARS-CoV-2 Infection. bioRxiv, 2020. doi: https://doi.org/10.1101/2020.12.08.416636, https://www.biorxiv.org/content/10.1101/2020.12.08.416636v1
- Peer reviewed and published scientific report.
Breton, Gaëlle, Pilar Mendoza, Thomas Hägglöf, Thiago Y. Oliveira, Dennis Schaefer-Babajew, Christian Gaebler, Martina Turroja, Arlene Hurley, Marina Caskey, and Michel C. Nussenzweig. 2021. “Persistent Cellular Immunity to SARS-CoV-2 Infection.” Journal of Experimental Medicine 218 (4). https://doi.org/10.1084/jem.20202515. https://rupress.org/jem/article/218/4/e20202515/211727/Persistent-cellular-immunity-to-SARS-CoV-2.