Since the worldwide outbreak of coronavirus disease 2019 (COVID-19) began, more than 76 million have been infected, with over 1.68 million deaths. The virus causing this pandemic is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), one of seven pathogenic coronaviruses to have hit the world so far. Even as the first vaccines roll out, the search for specific and safe treatments is still ongoing, as new variants arise, forcing new lockdowns in several regions.
Study aims
Coronaviruses target multiple tissues in the human host, including the lungs and upper airway cells, the intestinal tissues, the kidneys and the liver. They bind to the host cells via angiotensin-converting enzyme 2 (ACE2) receptors and perhaps other receptors such as neuropilin-1 (NRP1). Once the virus attaches to the ACE2 receptor, proteolytic cleavage occurs using host cell proteases. This generates an S2 fragment that is concerned with stimulating viral-host cell membrane fusion, allowing the virus to enter the cytosol.
Most potential therapies target this binding step. At present, some promising lead compounds include protease inhibitors and soluble ACE2 molecules that act as decoys, preventing the virus from engaging host cell receptors. The high rate of mutation of positive-sense RNA viruses gives them an advantage by allowing them to avoid the specific inhibition caused by these molecules.
The current study was aimed at understanding how to develop a broad-spectrum antiviral that targets fundamental aspects of the host-virus interaction. This could help to fight future coronaviruses as well. The current study looked into the possibility of using a palmitoylation inhibitor to treat SARS-CoV-2 infection.
Palmitoylation of spike necessary for new virions
Earlier reports showed that a protein called palmitoyltransferase ZDHHC5, along with its binding partner Golga7, interacted with the SARS-CoV-2 spike protein. Their research, which used affinity purification and mass spectrometry, suggested that the tail of the spike protein, which is in the cytosolic domain, undergoes palmitoylation after its generation within the host cell. This is similar to the process that occurs with the murine hepatitis virus (MHV), the spike protein of which must have C-terminal palmitoylation in order for viral replication to proceed successfully.
If palmitoylation is prevented, the spike protein cannot be assembled into new virions. The mutant spike protein was also unable to stimulate membrane fusion to form syncytia by cell-cell fusion, unlike the wild-type virus.
Multiple palmitoylation sites
In many coronaviruses that infect both human and murine cells, the C-terminal domains showed an enrichment of cysteine residues in the last 20 residues, near the membrane. With SARS-CoV-2, 10 of the first 20 residues are cysteine, allowing plenty of palmitoylation space.
The researchers used HEK293T cells, allowing them to express the SARS-CoV-2 spike protein tagged with a C9 epitope on the C-terminal end. They found that these cells contained a number of spike protein species, some full-length, some comprising the S2 subunit, and some multimers of the spike protein.
These cells were then cultured in the presence of a palmitate analog donor compound, called 15-hexadecynoic acid (15-HDYA). The researchers wanted to find out if this palmitate analog could be bound to the spike protein covalently, whether the full-length, S2 or the multimeric species.
They then used acyl-polyethylene glycol (PEG) exchange (APE), a method that involves switching out each spike palmitoyl group for a 5-kDa PEG mass tag. This would help to measure how much palmitoylation would occur on a per protein basis. They found that the spike protein could be modified on numerous sites. Looking at individual proteins, there were at least four palmitoyl groups.
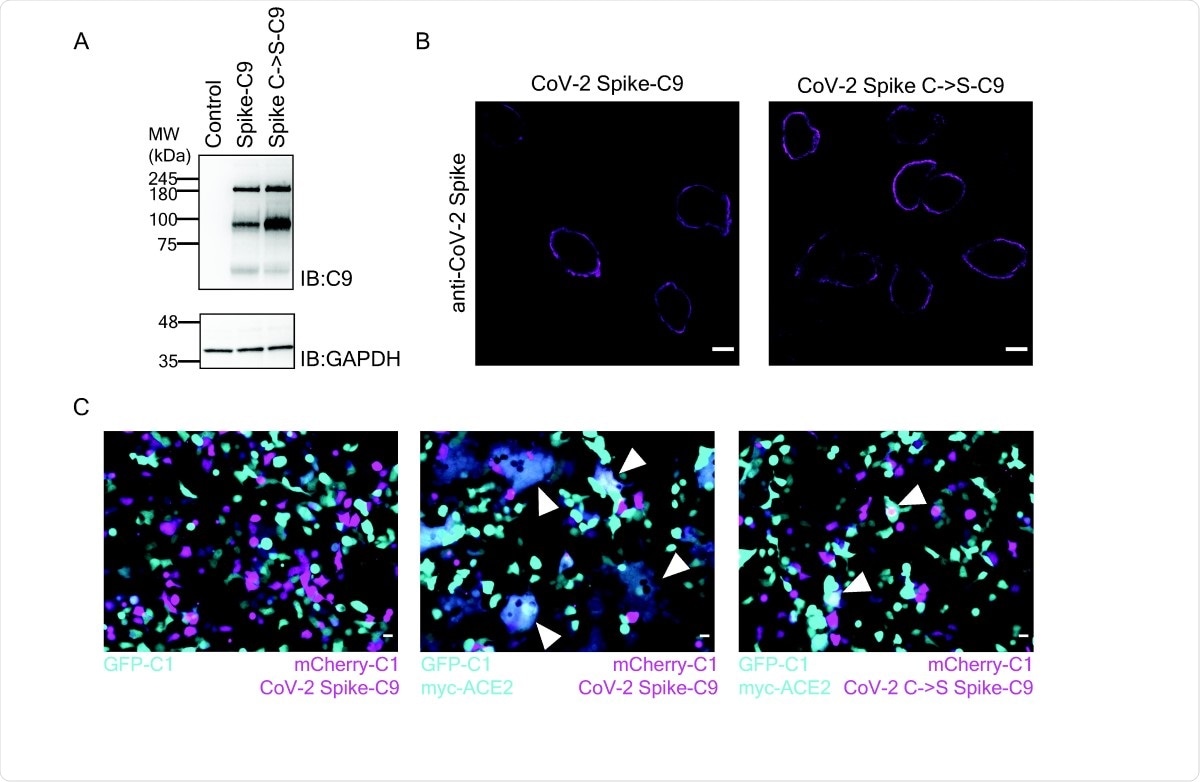
Palmitoylation of Spike is required for cell-cell fusion. (A) Western blot of SARS-CoV-2 Spike-C9 of the ten Cys to Ser mutant. (B) Immunofluorescence of SARS-CoV-2 Spike and the SpikeC->S-C9 mutant. Scale bars, 10µm (C) Co-culture of HEK cells expressing Spike-C9 and ACE2 form syncytium. HEK293T cells cotransfected with mCherry-C1 (magenta), and SARS-CoV-2 Spike-C9 (left and mid) or SARS-CoV-2 SpikeC->S-C9 (right) were co-cultured with cells transfected with EGFP-C1 (left, cyan) or and myc-ACE2 (mid, right). Scale bars, 20 µm. Arrowheads indicate fused cells.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Palmitoylation required for syncytia formation
Next, they used a technique to induce site-directed mutagenesis to convert all ten cysteine residues to serine instead. They used fluorescence microscopy to determine if the spike-C9 cells and cysteine>serine spike proteins were equally able to catalyze cell-cell fusion with cells that express ACE2. As expected, they found that numerous large syncytia were formed with spike-C9 but not with the cysteine>serine variant of the spike. “The results demonstrate that the Cys residues are required for the Spike protein to facilitate syncytium formation.”
Silencing of ZDHHC5 reduce viral plaque formation
In the human genome, there are 23 ZDHHCs (zinc finger Asp-His-His-Cys domain-containing palmitoyltransferases, of which only ZDHHC5 palmitoylates the spike protein of SARS-CoV-2. In order to find out if silencing this gene would be effective in limiting viral spread in culture, they used a less lethal virus, human CoV 229E, that was appropriate for work with biosafety level 2. This has a cytosolic tail that resembles that of SARS-CoV-2 and can be palmitoylated at multiple sites. Like the SARS-CoV-2 spike, the 229E spike is capable of ZDHHC5 palmitoylation.
Using a cell-based assay for 229E with silenced ZDHHC5, they found fewer plaques were formed relative to the controls. To determine if this was due to impaired infection or subsequent spread of the infection through the monolayer of cells, they incubated cells with the virus in liquid culture. This showed that ZDHHC5 is required, not for initial infection, but for the assembly and release of infectious viral particles.
Inhibition of FASN prevented SARS-CoV2 spike palmitoylation
There is no specific ZDHHC5 inhibitor at present, but the fatty acid synthase (FASN) inhibitor cerulenin blocks the addition of palmitoyl to NOD1 and NOD2, these being substrates of ZDHHC5. Since this is a toxic compound, they tested another FASN inhibitor molecule, called TVB-3166, and bromopalmitate, a non-specific inhibitor of ZDHHC enzymes. They found that spike palmitoylation was reduced for both viruses, while in plaque-forming unit assays, the number of plaques formed by 229E was 86% less.
FASN inhibitor increases MHV-infected mouse survival
The compound TVB-3166 can be given orally, and the researchers tested it in mice infected with the 229E virus. They found that while control mice showed signs of disease within two days of infection, which continued to progress, treated mice survived for longer periods and many survived. “The results demonstrate that reducing palmitoyl-CoA levels is beneficial during CoV infection as this reduction promotes survival in mice.”
Implications and future directions
The study therefore suggests that S2 subunit palmitoylation is essential for viral membrane fusion, in SARS-CoV-2, MHV and SARS viruses. In the MHV, indeed, the spike palmitoylation is needed to allow it to interact with the membrane protein that allow it to assemble the spike into the new viral particles. Adding TVB-3166, whether in cell assays or in infected mice, reduced the production of new infectious virus particles and increased mouse survival.
The mechanism of action of this molecule points to the role of altered lipid metabolism in coronavirus infections. Some signs of this are changes in apolipoproteins and serum lipids, as well as increased free fatty acid levels. These lipid changes may promote viral replication. There is also a linoleic acid-binding pocket in the ectodomain of SARS-CoV-2, as well as other pathogenic coronaviruses, indicating the need for research on how fatty acids and lipid metabolism are linked to coronavirus infection.
Further studies to test the possible therapeutic role of TVB-2640, a related FASN inhibitor that is already in clinical trials for cancer and non-alcoholic liver disease, is orally available, and well-tolerated in patients. Since FASN is a host enzyme, viral escape mutations are unlikely to emerge. “The identification and approval of an orally available pan-CoV treatment or prophylaxis would be beneficial in the short-term and the future against other zoonotic CoVs.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources