As severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to wreak its havoc, antibodies that neutralize the virus and protect against infection have become an important focus of research. However, there is a concern as to whether these antibodies can actually enhance the disease.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Antibody-mediated disease enhancement
With the earlier outbreaks of Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome coronavirus (SARS), the phenomenon of antibody-dependent enhancement (ADE) of disease was observed. This has become a major concern relating to the safe administration of antibody prophylaxis, antibody therapy and antibody-based vaccines against the ongoing coronavirus disease 2019 (COVID-19) pandemic.
Individuals with severe COVID-19 tend to develop antibodies earlier than those with mild disease. This has also led to the suspicion that antibodies may contribute to the intensity of disease.
Disparity between in vitro and in vivo ADE
In the current study, there was enhanced infection in the presence of antibodies to the virus in vitro. However, when mouse experiments were carried out, as well as a nonhuman primate model, this finding was not reproduced, with either SARS-CoV-2 or SARS-CoV.
The researchers looked for either severe lung disease due to immune infiltrates and inflammation, resulting from antibody-mediated pro-inflammatory pathways or from antigen-antibody complexes. They also looked for increased replication of the virus in the respiratory tract. However, they found that mice showed neither of these changes significant of ADE.
Again, 30 monkeys were given the antibodies to the receptor-binding domain (RBD) or the N-terminal domain (NTD) of the SARS-CoV-2 spike protein that appeared to be connected to ADE in vitro. However, only two monkeys showed a higher level of lung inflammation in the presence of low or no viral antigen in the lung. Only one had indications of ADE, in the lung histologic examination and the levels of cytokines in the bronchoalveolar lavage (BAL) fluid. Thus, multiple factors appear to be at work, beyond simple ADE.
These preclinical results indicate that SARS-CoV-2 antibody treatments or the induction of SARS-CoV-2 antibodies by vaccination have a low likelihood of exacerbating COVID-19 disease in humans.”
Explanations for observed differences
The lack of correlation between the in vitro ADE activity of these antibodies during in vivo experiments has been observed with polyclonal serum antibodies in earlier experiments.
The researchers say this incongruity could be due to the absence of productive SARS-CoV-2 infection in macrophages and other phagocytic cells, unlike with MERS-CoV during ADE. In the latter case, the ADE is mediated by FcγR regions of the antibody. With SARS-CoV and SARS-CoV-2, however, RBD antibodies may lead to FcγR-dependent virus uptake of SARS-CoV-2 in vitro but not in vivo, due to abortive infection of macrophages.
Antibody effector functions may prevent ADE
Another proffered explanation is that in vivo, antibody effector functions may target the virus, preventing its replication. For instance, the antibodies may tag infected cells or viral particles, for phagocytosis, or may cause antibody-mediated or direct cytotoxicity that leads to the destruction of the infected cells or viruses. This is mediated by the Fc portion of the antibodies, which may be a vital part of their protective function.
Antibody effector functions may contribute to the outcome in vivo, but not be accounted for in SARS-CoV-2 neutralization assays in vitro.”
This is borne out by the fact that the NTD antibody DH1052 is known to have ADE effects and to be non-neutralizing, but was capable of reducing the titers of infectious virus in the lungs of mice exposed to a viral challenge. This antibody binds to mouse FcγRs, which suggests that the Fc receptor could trigger antibody effector functions to reduce the titer of the infectious virus. This aspect requires further study.
Different or no FcγRs
In contrast to the conventional ADE mediated by FcγR-dependent viral uptake, SARS-CoV-2 RBD antibodies seem to use FcγRIIb or FcγRI. This may mean that the FcγRs that are involved in ADE in vitro with MERS or SARS-CoV are different from those occurring in SARS-CoV-2. Also, the researchers found that non-neutralizing NTD antibodies caused FcγR-independent ADE in two different cell types expressing the host cell viral receptor, angiotensin-converting enzyme 2 (ACE2). This could be related to the higher preference of NTD antibodies for the ‘down’ conformation of the 3 RBDs of the trimeric spike.
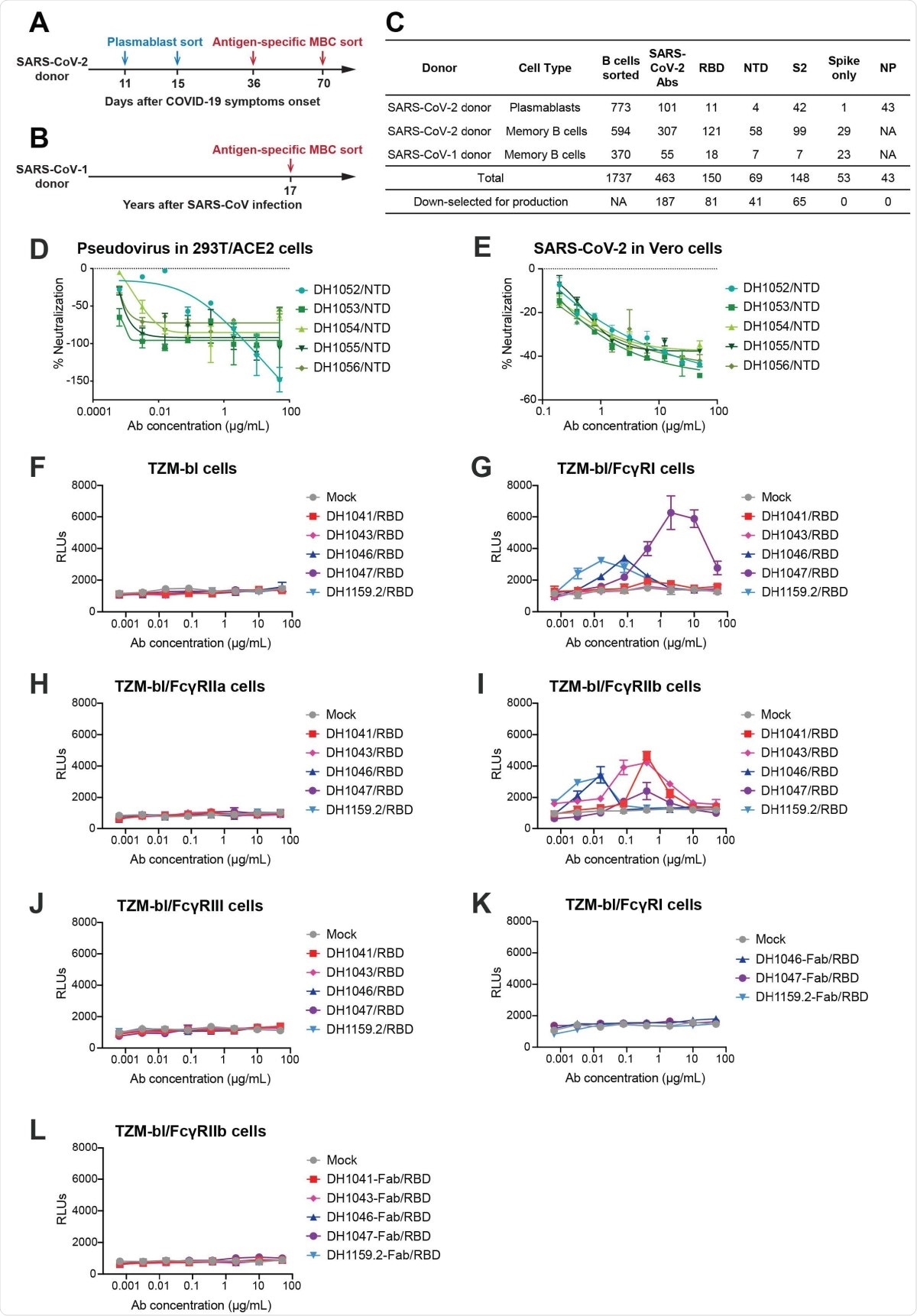
SARS-CoV-2 receptor-binding domain (RBD) and N-terminal domain (NTD) antibodies mediate FcγR-dependent and FcγR-independent enhancement of SARSCoV- 2 infection respectively. (A-B) Timeline of blood sampling and antibody isolation from convalescent SARS-CoV-2 and SARS-CoV-1 donors. Plasmablasts and/or antigen-specific memory B cells (MBC) were sorted from a (A) SARS-CoV-2 infected individual (SARS-CoV-2 donor) and a (B) 2003 SARS survivor (SARS-CoV-1 donor). (C) Summary of number and specificity of antibodies isolated from each donor. D-E) Fc?R-independent SARS-CoV-2 infection-enhancement mediated by nonneutralizing NTD antibodies. In vitro neutralization curves for NTD infection-enhancing antibodies against (D) pseudotyped SARS-CoV-2 D614G in 293T-hACE2 cells, and (E) replication-competent nano-luciferase (nLuc) SARS-CoV-2 in Vero cells. (F-J) FcγR-dependent SARS-CoV-2 infection-enhancement in ACE2-negative cells mediated by neutralizing RBD antibodies. Pseudotyped SARS-CoV-2 incubated with RBD antibodies or mock medium control were inoculated on (F) parental TZM-bl cells, and TZMbl cells stably expressing human FcγR receptors (G) FcγRI, (H) FcγRIIa, (I) FcγRIIb or (J) FcγRIII. (K-L)The effect of RBD antibody fragment antigen-binding regions (Fabs) on pseudotyped SARS-CoV-2 D614G infection were tested in (K) FcγRI-expressing TZM-bl cells and (L) FcγRIIb-expressing TZM-bl cells. Relative luminescence units (RLUs) were measured in cell lysate at 68-72 hours post-infection. Upward deflection of RLUs in the presence of antibody indicates FcγR-mediated infection. Three or four independent experiments were performed and representative data are shown.
What are the implications?
The therapeutic antibody LY-CoV555 was found to be ineffective in treating hospitalized patients with COVID-19. The reason may be the presence of high titers of ADE-linked antibodies, that might have counteracted the protective effects of the neutralizing antibody.
Currently, ADE has not been demonstrated with any of the vaccines developed against COVID-19 so far, nor with the use of therapeutic antibodies or convalescent serum. However, when neutralizing antibodies are suboptimal, the severity of COVID-19 is likely to be greater. Thus, vaccine trials must be followed up to detect vaccine associated enhanced disease (VAED).
Given that the patient from whom many of the antibodies in the current study were obtained did not develop severe COVID-19, and the lack of correspondence between ADE in vitro and in vivo, the researchers concluded, “These results suggest that antibody-induced enhancement of infection is a rare possibility but will not likely be a biologically relevant adverse effect following COVID-19 vaccination in humans.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources