In the pursuit of effective antiviral drugs to counter the current coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), few stones have been left unturned. One of the earliest drugs to be approved by the US Food and Drug Administration (FDA) was remdesivir, even though its clinical trial results showed non-remarkable efficacy, and came from a single study.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Remdesivir
Remdesivir is a phosphoramidate prodrug, and requires conversion through enzymatic action to its active form, the nucleoside triphosphate of remdesivir (NTP; GS-443902). This is incorporated into the viral RNA and ends transcription as well as replication by inhibiting the viral RNA-dependent RNA polymerase.
This is accomplished by enzymes that are highly expressed in liver tissue but poorly in alveolar type 2 cells (AT2). This may explain why only a single double-blind, placebo-controlled randomized clinical trial (RCT) showed even slight efficacy against SARS-CoV-2, since the lungs are the primary site of attack in this condition.
Other RCTs failed to demonstrate significant improvements in mortality with the use of this drug.
The metabolism of remdesivir by the liver may cause toxicity to the liver cells, which limits dose escalation to potentially more effective dosage ranges. Moreover, remdesivir is a hydrophobic drug, and must be formulated with excipients in complex form. The latter could impair renal function.
And finally, remdesivir can only be given via the intravenous route, making it non-feasible for outpatient use. This means it cannot be tested in early COVID-19, for its efficacy in preventing disease progression or the development of clinical disease following SARS-CoV-2 infection.
Finally, escape mutations on RdRp may allow the virus to surmount the inhibitory effects of remdesivir.
GS-441524 may be more effective
In view of the obvious drawbacks of remdesivir, its parent compound GS-441524 has been suggested to be a better choice in treating this condition. One reason is that it is the dominant metabolite found in plasma after the intravenous infusion of remdesivir, and persists the longest in preclinical studies. Its half-life in humans in is over 24 hours.
GS-441524 is converted to GS-443902 in the body by nucleoside kinases (probably adenosine kinase), enzymes found in a range of tissues. In this, too, it is unlike remdesivir. Besides, its safety profile is superior, allowing for the use of higher doses without dose-related liver toxicities. This enables higher concentrations of the final bioactive metabolite GS-443902.
In cell cultures, the activity of GS-441524 against SARS-CoV-2 infection has been found to be powerfully inhibitory. The 50% effective concentrations are comparable to that of remdesivir itself, in vitro.
In cats, naturally infected with the feline coronavirus (FCoV), and showing feline infectious peritonitis (FIP), the use of GS-441524 subcutaneously has shown a cure rate of 96%. In mouse studies, using either SARS-CoV-2 or murine hepatitis virus (MHV), which is closely related to the former, GS-441524 has been found to reduce viral loads in affected organs with encouragingly low toxicity levels.
These results have driven the current study on the pharmacokinetics of GS-441524 in dogs, since they indicate that this drug could potentially be developed as an orally available agent against COVID-19 on an outpatient basis.
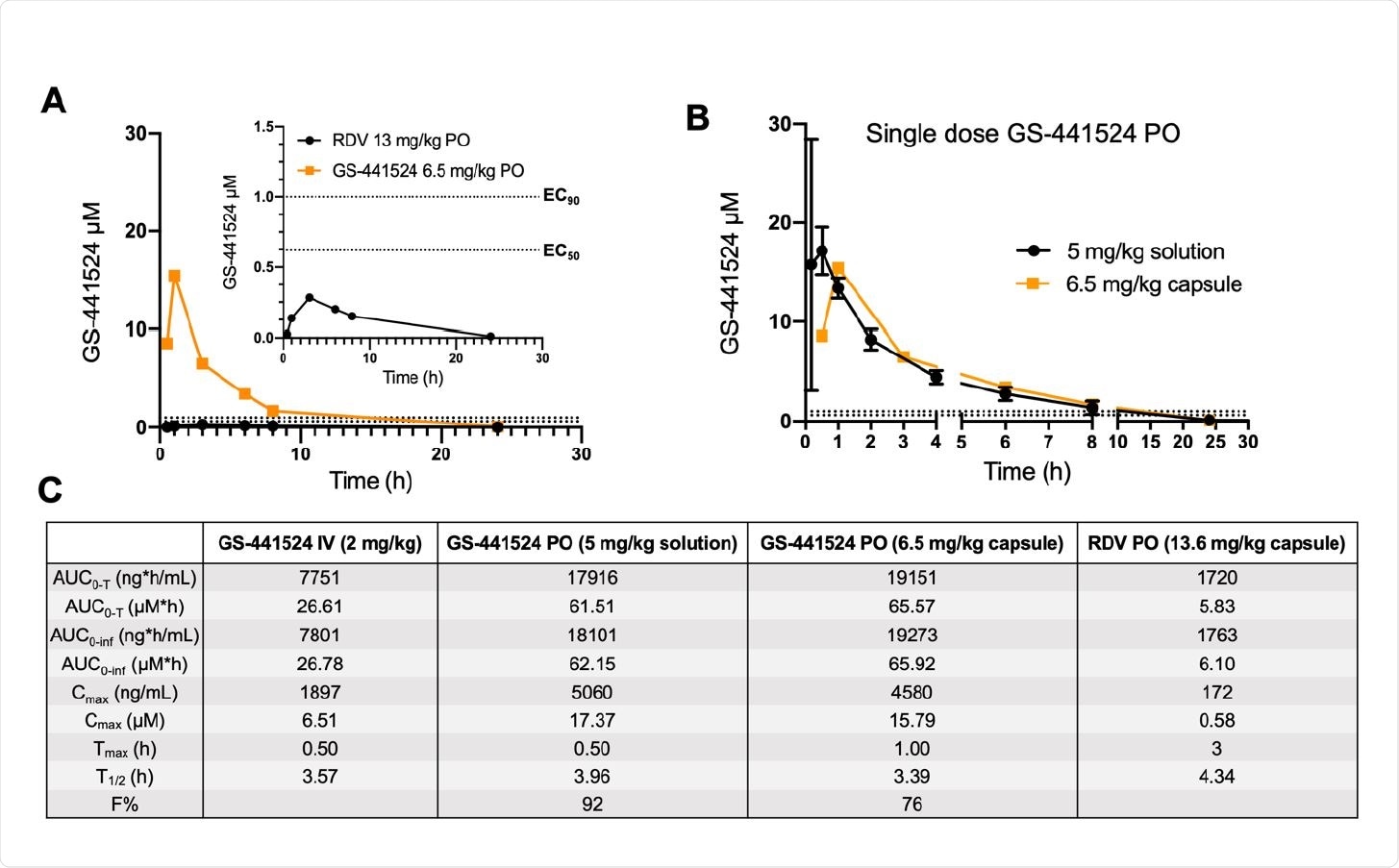
Plasma c 187 oncentrations of GS-441524 following a single oral dose of remdesivir or GS-441524 in dogs. (A) Head-to-head PK comparison following a single equimolar dose of remdesivir (black, 13.6 mg/kg) and GS-441524 (orange, 6.5 mg/kg) in male beagle dogs (N=1 per compound). Both compounds were administered in capsule form. Plasma concentrations of GS-441524 following compound administration are plotted for the following timepoints (h): 0.5, 1, 3, 6, 8, 24. A focused view of GS- 441524 concentrations following oral administration of remdesivir is shown in top right corner. (B) Comparison of plasma concentrations of GS-441524 following oral administration as a solution (black, 5 mg/kg; N=3) or as a capsule (orange, 6.5 mg/kg; N=1). (C) Mean PK parameters following various routes of administration of GS-441524 and RDV. Raw values for GS-441524 dosed IV and PO dosed as a solution are adapted from NCATS OpenData Portal and have been re-calculated to match the sampling timeframe of the capsule studies (T=0.5-24 h). All PK parameters were calculated using PKSolver 2.0. In panels A and B, dotted lines correspond to EC50 (bottom) and EC90 (top) values reported for GS-441524 in SARS-CoV-2-infected Calu3 cells (19).
Low liver metabolism
The researchers established that when dogs were given either oral GS-441524 or oral excipient-free remdesivir, the plasma concentrations of the former were 25-fold higher. Since the former is the metabolite of the latter, its concentration peaked at one hour following administration, but at three hours after administration of remdesivir.
The shift in values may have been due to the systemic release of remdesivir following the model described with this class of compounds. That is, the prodrug is rapidly removed from circulation by the liver, and then accumulates in two forms – the active nucleoside triphosphate and the hydrolyzed nucleoside. The latter form is slowly released into the systemic circulation.
Even then, the concentrations of GS-441524 following remdesivir administration are below the EC50 values required against SARS-CoV-2. Moreover, this dose cannot be used long-term in humans due to the associated liver toxicity. This rules out the possibility of using oral remdesivir to attain therapeutic levels of GS-441524 in COVID-19.
Oral bioavailability
Conversely, when directly administered, GS-441524 reaches EC50 values within an hour, and were maintained for eight hours or more, indicating high oral absorption. This confirms earlier studies showing the oral bioavailability of 85% in dogs. Even when used without excipients, at a slightly higher dosage, the high solubility of GS-441524 allowed a high oral bioavailability of 76%.
These data hint at the feasibility of using an excipient-less pill formulation for GS-441524 for outpatient treatment.”
What are the implications?
The number of dogs to which excipient-free GS-441524 was administered did not capture individual variability in formulation-derived dosage. Also, dogs typically have higher oral bioavailability compared to other early experimental species. This may be due to their possessing a paracellular nuclear transporter lacking in humans and non-human primates.
This caveat means that humans may have a lower oral bioavailability than indicated from the findings of this study. Despite this, “As a prodrug inhibitor of the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp), GS-441524 is aptly poised to demonstrate consistent efficacy among new mutations of SARS-CoV-2, as RdRp is much less susceptible to efficacy-altering mutations than is the spike protein.”

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.