Some people with coronavirus disease 2019 (COVID-19) develop symptoms, while others do not. In high-risk populations, like the elderly and those with underlying health issues, may experience more severe symptoms.
A new study by researchers at the Université de Lyon, France, noted that some COVID-19 symptoms like heart arrhythmias, loss of smell, gastrointestinal problem, and impaired oxygen uptake may be explained by a potential interference of ORF7b, which is an accessory protein found in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The study, published in the pre-print journal medRxiv*, highlights the potential causes of some COVID-19 symptoms. Early in the pandemic, the hallmark symptoms of COVID-19 include cough, fever, and shortness of breath. As the pandemic has evolved, more symptoms have emerged, such as loss of smell and taste, headache, intestinal issues, and abdominal pains.
SARS-CoV-2 structure
The SARS-CoV-2 contains a spike protein (S) that binds with the host cell’s angiotensin-converting enzyme 2 (ACE2) receptor, which acts as the virus’s cellular gateway to infect the cell.
Coronaviridae is enveloped, single-stranded RNA viruses that infect birds and mammals, including humans. The current pandemic is one of the largest outbreaks in history, infecting more than 106.61 million people worldwide and causing 2.33 million have died.
Coronavirus replication is facilitated by many highly conserved viral proteins. The viruses also encode accessory genes, a group believed to play pivotal roles in virus replication and pathogenesis.
The SARS-CoV-2 has accessory proteins, which are necessary for viral replication and can mediate host response to the virus, affecting pathogenicity and virulence.
The ORF7b is an accessory protein of SARS-CoV-2, showing more than 93 percent sequence homology with a bat virus 7b protein. This protein is a putative viral accessory protein encoded on subgenomic RNA 7. Previous studies have shown that ORF7b is not only an accessory protein but a structural component of the SARS-CoV-2 virus.
The study
In the current study, the researchers used cell-free synthesized ORF7b and revealed that ORF7b assembles into stable multimers. Also, the ORF7b sequence shows a transmembrane segment. Hence, the team believes that this accessory protein has the potential to interfere with important cellular processes that involve leucine-zipper formation.
Leucine zippers work by regulating the rhythm of the heart via the multimerization of phospholamban in cardiomyocytes. Next, epithelial cell-cell adhesion relies on E-cadherins.
The team noted that the most common symptoms of SARS-CoV-2 infection, like heart arrhythmias, loss of smell, intestinal problems, and impaired oxygen uptake, can be explained by a possible interference from ORF7b.
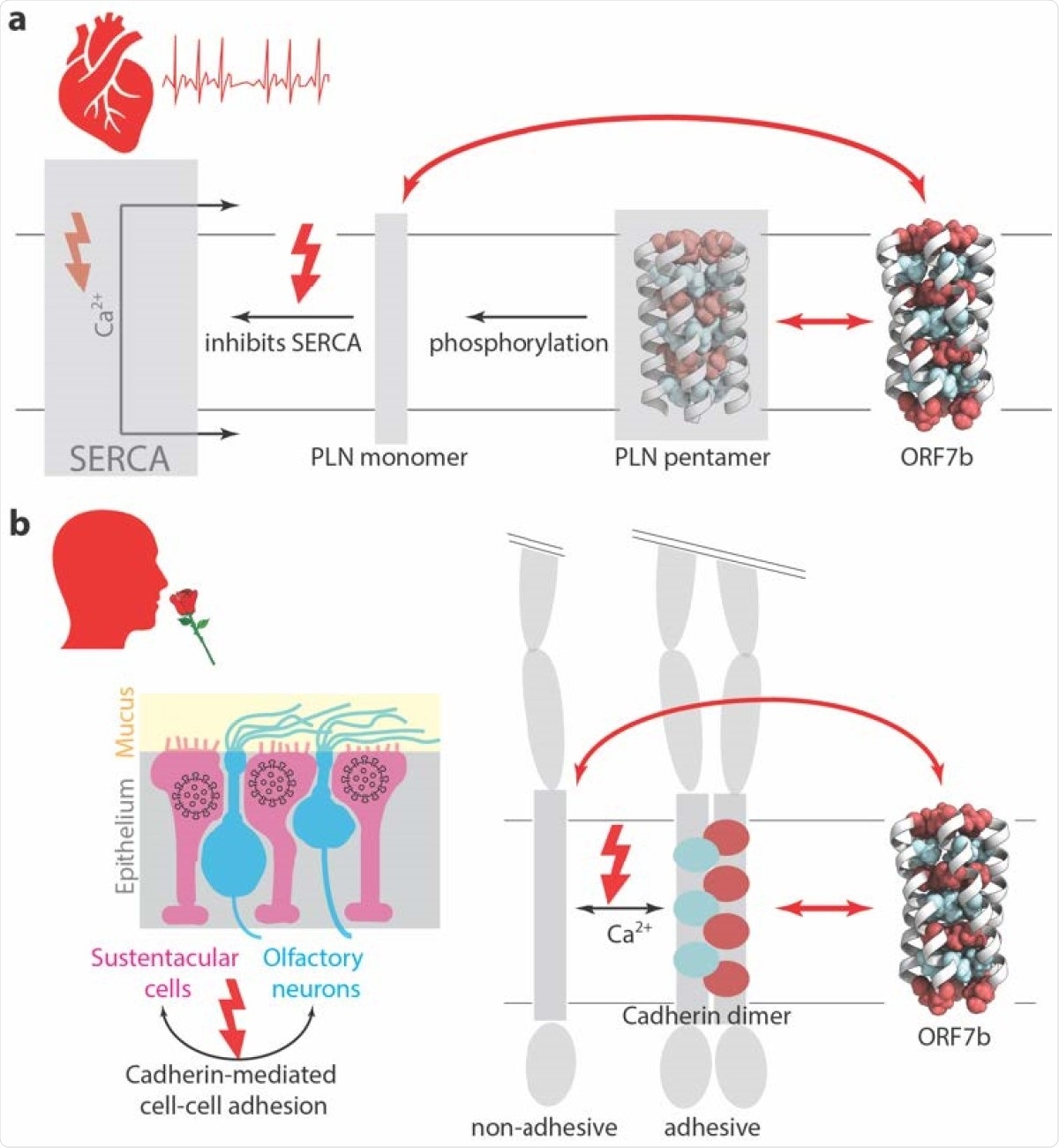
ORF7b has the potential to interfere with cellular functions. a) In the heart muscle, SERCA catalyzes Ca2+ transport across the membrane. PLN is an inhibitor of SERCA, and thus regulates Ca2+ transport, and ultimately heart muscle contraction and heart rhythm. PLN binds to SERCA as a monomer, that is formed in a phosphorylation-dependent manner. ORF7b has the potential to interact, via its leucine zipper, with PLN monomers. b) Olfactory neurons present cilia to the mucus. They are supported by sustentacular cells in the epithelium. Only these support cells can be entered by the virus. Cell-cell adhesion between same and different cell types is mediated by E-cadherin. For E-cadherin to be adhesive, it must dimerize, including via the leucine zipper transmembrane domain. ORF7b has the potential to interefere with adhesion by interaction of its leucine zipper with the one from the E-cadherin transmembrane domain. Cartoon of olfactory endothelium adapted from (56).
The team added that ORF7b forms robust multimers, which are stabilized by a leucine zipper. They believe that the strong relationship between the ORF7b and the leucine zipper makes the viral protein interact with transmembrane leucine zipper proteins, making ORF7b a major suspect or culprit for interfering with cellular functions commonly seen in SARS-CoV-2 infection.
More precisely, we propose that ORF7b is a suspect as causative agent for heart arrythmia and loss of smell, and advance possible molecular bases,” the team concluded.
Drugs that act on ORF7b by controlling its self-interactions with cellular proteins may become an important tool or weapon in the battle against COVID-19.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Source:
Journal reference: