Vaccines may come in several forms, one of the most common being live-attenuated. Live-attenuated vaccines are a weakened form of the virus, created by genetic modification, and usually provide long-lasting immunity. Many research groups and companies have been working tirelessly since the outbreak of COVID-19 to develop vaccines by a number of routes, though no live-attenuated vaccine has thus far been approved.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Vaccines
Unlike any vaccine approved for use before them, two of the three most popular COVID-19 vaccines currently approved (Moderna and Pfizer-BioNTech) are based on mRNA delivery by a lipid nanoparticle. The mRNA payload is delivered into the cytoplasm of cells to utilize the cell's own machinery to produce antigens belonging to the SARS-CoV-2 virus, specifically for sections of the spike protein of the virus that would interact with receptors on host cells. As implied, this technology is relatively emergent with regards to wide-scale production, and the complexity and cost of production and transport is much higher than more classically produced vaccines. Additionally, numerous adjuvants may be added that induce an immune response, adding to the complexity of formulation and storage.
The third of the currently deployed vaccines, by Oxford-AstraZeneca, uses an adenovirus vector sourced from the chimpanzee. One of the advantages of using a viral vector such as this is that it stimulates an immune response without the need for adjuvants. The vector contains dsDNA that utilizes the machinery of the cell in the nucleus to transcribe a modified form of the full-length spike protein. This vector is non-replicating, meaning that it generally only confers temporary immunity. Additionally, the dosing requirements are therefore higher per dose of vaccine, with hundreds of thousands times more viral particles being required compared to live-attenuated vaccines. Subsequently, more intense side effects are often reported from these vaccines, with early trials of the Oxford-AstraZeneca COVID-19 vaccine reporting numerous side-effects.
The live-attenuated measles vector vaccine platform is one of the safest and effective available, having been the basis for a number of common vaccines over the last several decades. Since it is self-replicating, the immunity conferred is long-lasting, and the production, storage, and transport infrastructure needed for wide-scale distribution are already in place.
How was the vaccine designed?
The group selected the full-length spike protein of SARS-CoV-2 as the main antigen expressed by the vector, and modified it to be better transcribed by the machinery of human cells and be more stable in its pre-fusion form, similarly to the Moderna vaccine. Additionally, the tail of the spike protein was modified to prevent it from being recycled away from the plasma membrane, keeping it at the surface of host cells for longer periods, where it can be detected by immune cells.
In pursuit of developing a wide-spectrum vaccine for SARS-like coronaviruses (CoVs) a version of the vaccine expressing one particular highly conserved subunit of the spike protein was also designed, though this form proved to exhibit much lower cellular responses and so was dropped from further analysis.
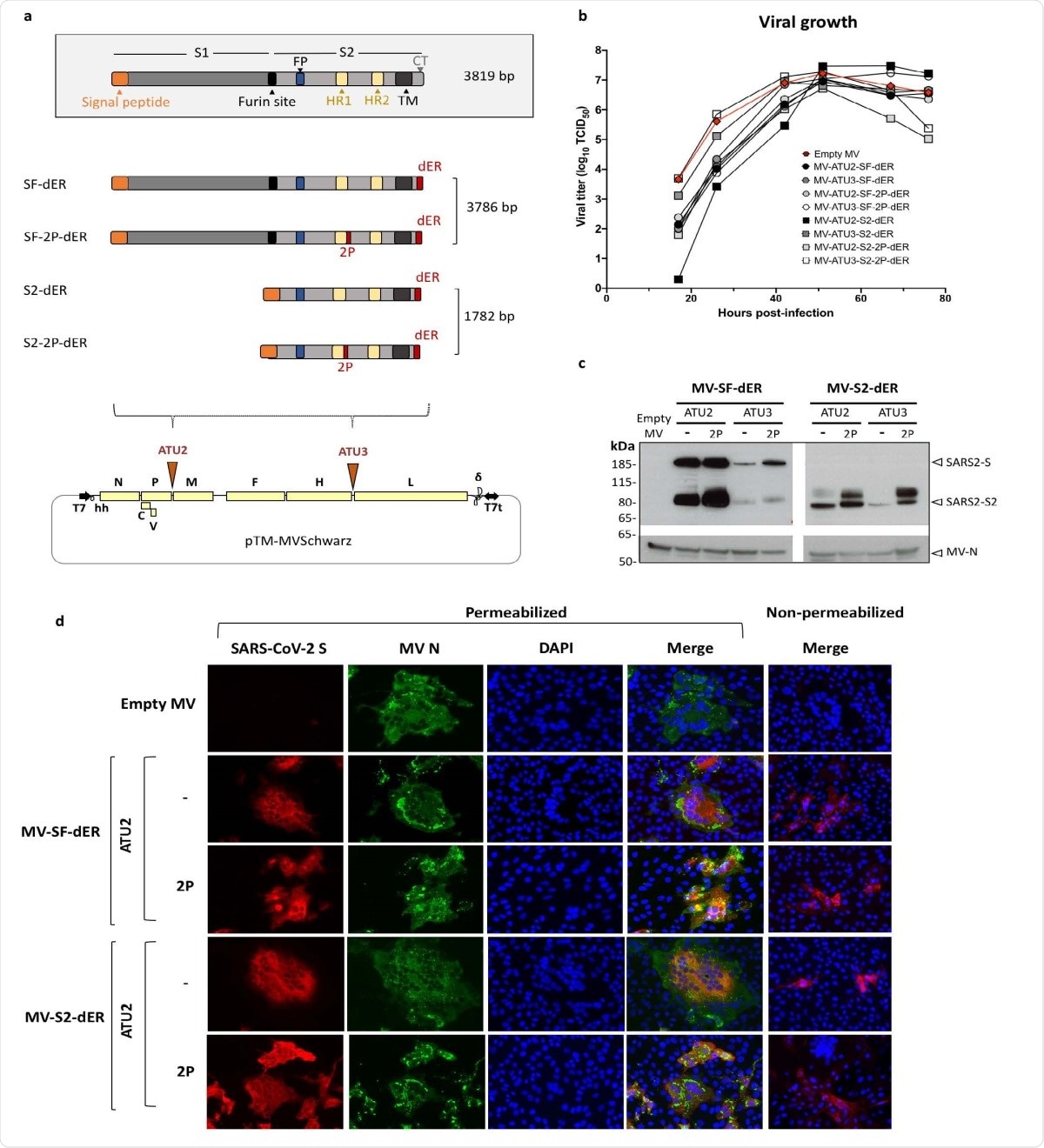
Schematic of S gene constructs and characterization of S-expressing rMVs. a) The native S gene of SARS-CoV-2 with notable domains indicated in color boxes relative to the S gene constructs cloned into the MV vector. 2P and dER modifications indicated by the red boxes. All S constructs were cloned into either the second (ATU2) or third (ATU3) additional transcription units of pTM-MVSchwarz (MV Schwarz), the MV vector plasmid. The MV genome comprises the nucleoprotein (N), phosphoprotein (P), V and C accessory proteins, matrix (M), fusion (F), hemagglutinin (H) and polymerase (L) genes. Plasmid elements include the T7 RNA polymerase promoter (T7), hammerhead ribozyme (hh), hepatitis delta virus ribozyme (?), and T7 RNA polymerase terminator (T7t). b) Growth kinetics of rMV constructs used to infect Vero cells at an MOI of 0.1. Cell-associated virus titers are indicated in TCID50/ml. c) Western blot analysis of SARS-CoV-2 S protein in cell lysates of Vero cells infected with the rMVs expressing SF-dER or S2-dER from either ATU2 or ATU3, with or without the 2P mutation. d) Immunofluorescence staining of Vero cells infected with the indicated rMVs 24 h after infection. Permeabilized or non887 permeabilized cells were stained for S (red), MV N (green) and nuclei (blue).
To demonstrate the immunization achieved, mice given two doses of the vaccine were exposed to a mouse-adapted form of the SARS-CoV-2 virus 80 days after administration and their lungs were examined for any traces of the virus three days later. No virus was detected, though evidence of viral RNA lingered, suggesting that though some replication was taking place, the virus's progeny was effectively neutralized. The experiment was repeated, this time giving only one dose of the vaccine. In this case, viral particles were detected in the lungs, though at lower levels compared to the control.
Overall the authors estimate an efficacy rate of 90% from the first dose of the vaccine, and 100% following the second. Long-lasting memory B- and T- cells are stimulated by the vaccine, and spike protein-specific IgG titers persisted in mice for up to three months, suggesting that it could confer long-term immunity.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Namprachan Frantz, Phanramphoei, Aleksandr Barinov, Claude Ruffié, Chantal Combredet, Valérie Najburg, Samaporn Teeravechyan, Anan Jongkaewwattana, Matthieu Prot, Laurine Conquet, Xavier Montagutelli, Priyanka Fernandes, Hélène Strick-Marchand, James Di Santo, Etienne Simon-Loriere, Christiane Gerke, Frédéric Tangy (2021) A measles-vectored COVID-19 vaccine induces long-term immunity and protection from SARS-CoV-2 challenge in mice. bioRxiv preprint server. https://doi.org/10.1101/2021.02.17.431630, https://www.biorxiv.org/content/10.1101/2021.02.17.431630v1
- Peer reviewed and published scientific report.
Frantz, Phanramphoei N., Aleksandr Barinov, Claude Ruffié, Chantal Combredet, Valérie Najburg, Guilherme Dias de Melo, Florence Larrous, et al. 2021. “A Live Measles-Vectored COVID-19 Vaccine Induces Strong Immunity and Protection from SARS-CoV-2 Challenge in Mice and Hamsters.” Nature Communications 12 (1): 6277. https://doi.org/10.1038/s41467-021-26506-2. https://www.nature.com/articles/s41467-021-26506-2.