SNX-5422 is a heat shock protein 90 (Hsp90) inhibitor currently undergoing clinical trials as a cancer therapeutic. A recent study, released on the bioRxiv* preprint server, examines the applicability of repurposing the drug towards severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication inhibition.
Many of the coronavirus disease 2019 (COVID-19) interventions developed so far are aimed at severe or critical disease and generally require intravenous delivery, typically in a hospital setting. Additionally, despite the demonstrated efficacy of vaccines, delays surrounding wide-scale adoption and distribution leave large portions of the global population without immunity, particularly in lower-income regions.
An early-therapy prophylactic that could be administered to patients to dampen the progression of disease severity and lead to better outcomes with lower rates of hospitalization would ease the burden on COVID-19 treatment and problems associated with vaccine distribution.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
How was the influence of SNX-5422 established?
Hsp90 is a cellular chaperone protein involved in cellular homeostasis and the proper folding of virally encoded proteins. It has additionally been implicated in systemic and pulmonary inflammation, frequently an aspect of concern in severe COVID-19 cases.
Human lung epithelial cells and African green monkey kidney cells were exposed to SARS-CoV-2 and treated with SNX-5422. Treated cells bore fewer viral nucleocapsid particles, exhibited less viral RNA shedding, and a reduction in the number of cell-free viral genomic copies and viral titers.
Concentrations of only 2.3 μM, 0.2 μM, and 0.4 μM were required to achieve a 50% inhibitory concentration with regard to each of these parameters, respectively, while the cytotoxic concentration of SNX-5422 towards these cells was determined to be around or over 100 μM. This demonstrates the high specificity of SNX-5422, with minimal off-target interactions.
The interactions of SNX-5422 with host cellular machinery were also characterized using a human primary tracheobronchial epithelial cell model sourced from three separate donors. Total cellular RNA was sequenced, and genetic expression was determined to have been altered. In total, 1,361 genes were differentially expressed, with 470 being upregulated while 891 were downregulated. The four most upregulated and the 16 most downregulated genes were investigated further, with the most downregulated being revealed to be involved in cytokine generation, regulation of cellular inflammation and proliferation, and antigen presentation. The upregulated genes were involved in cellular adhesion and the regulation of proliferation and apoptosis.
Since viruses rely on host machinery to replicate, the machinery itself may be an attractive target in anti-viral strategies. Viruses are unlikely to develop resistance against such a mechanism, though interfering with normal cellular function could prove disastrous. The authors address these concerns by pointing out the high selectivity-index exhibited by SNX-5422 towards suppressing viral replication with minimal off-target effects, in addition to advising that the prophylactic is intended to be administered to those with mild or no symptoms only.
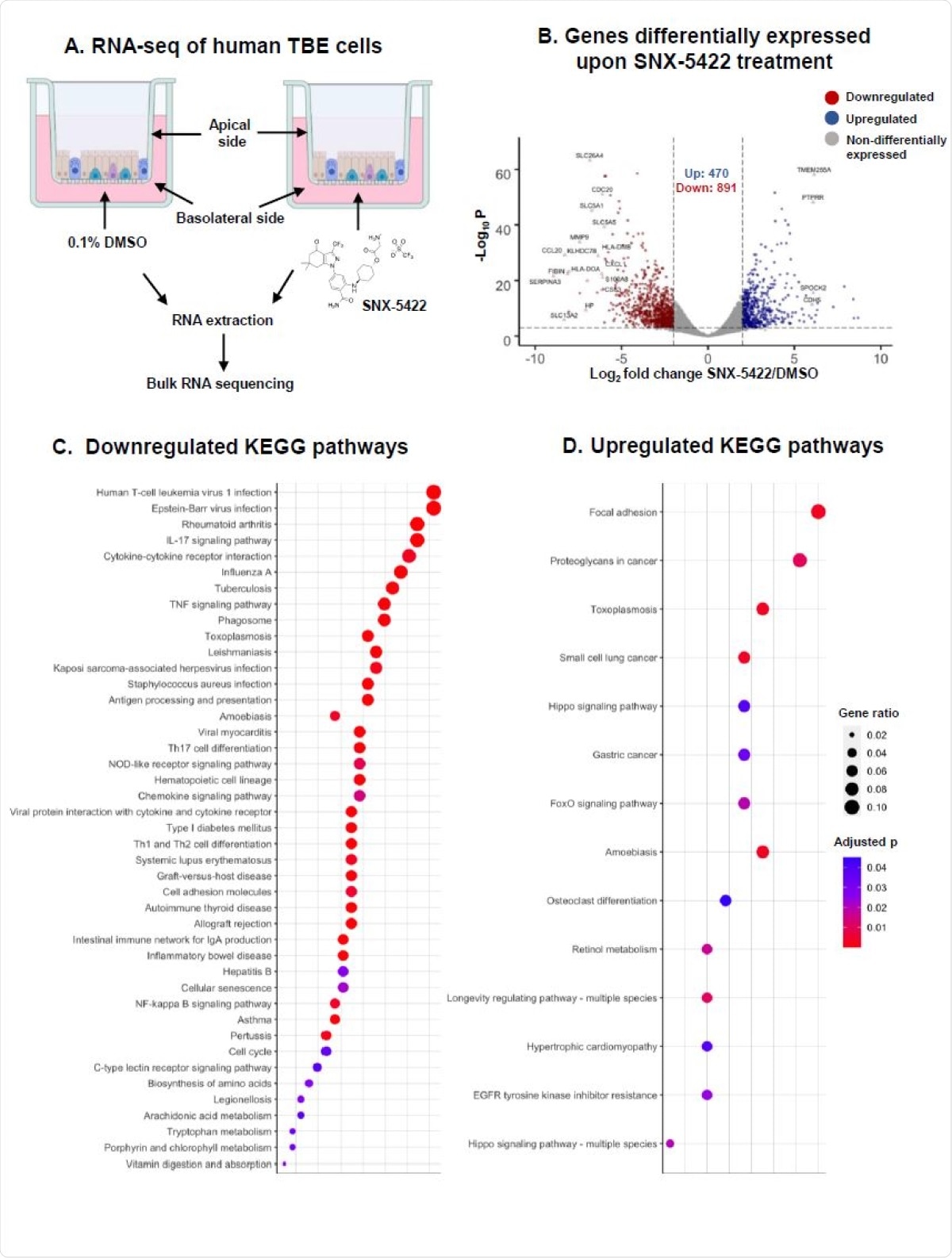
SNX-5422 treatment alters gene expression of human tracheobronchial epithelial (TBE) cells. (A) Schematic diagram illustrating treatment of human TBE cells. TBE cells from 3 independent healthy donors were cultured in air-liquid interface and treated with either 0.1% DMSO (drug-vehicle) or 1µM SNX-5422 reconstituted in DMSO, for 48hrs. RNA was then extracted from the cells and bulk RNA sequencing was performed. (B) Volcano plot demonstrating protein coding genes altered upon SNX-5422 treatment of human TBE cells. Red, blue and grey indicates downregulated, upregulated and non-differentially expressed genes, respectively. Differentially expressed genes (DEGs) were defined as protein coding genes with a Log2 fold change >2 between the drug-treated and control groups and adjusted p value <0.001. Open symbols represent genes with Log2 fold change >6 between the drug-treated and control groups. Cellular pathway overrepresentation analysis was performed with DEGs with a normalized average read count across donors and experimental conditions =300. (C) Downregulated and (D) Upregulated Kyoto Encyclopedia of Genes and Genomes (KEGG)pathways with an adjusted p value< 0.05 were reported. Dot size represented gene ratio and color schema represents adjusted p values.
What is the significance?
Hyperinflammation is strongly associated with poor COVID-19 outcomes, with patients exhibiting higher levels of circulating cytokines being under much greater risk of developing acute respiratory distress syndrome. The group has demonstrated that treatment with SNX-5422 results in downregulated inflammatory pathways and other genes associated with poor COVID-19 outcomes, altering the expression of host responses that normally become imbalanced by the actions of SARS-CoV-2. Future human trials will reveal the applicability of the COVID-19 preventative, potentially carving a path for the development of broad-spectrum anti-viral prophylactics.
Early treatment with SNX-5422 could potentially mitigate the inflammation induced by SARS-CoV-2, improving long-term outcomes and lowering the hospitalization rate.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Goswami, Ria, Veronica S. Russell, Joshua J. Tu, Philip Hughes, Francine Kelly, Stephanie N. Langel, Justin Steppe, Scott M. Palmer, Timothy Haystead, Maria Blasi, Sallie R. Permar (2021) Oral Hsp90 inhibitor, SNX-5422, attenuates SARS-CoV-2 replication and dampens inflammation in airway cells. bioRxiv preprint server. doi: https://doi.org/10.1101/2021.02.23.432479, https://www.biorxiv.org/content/10.1101/2021.02.23.432479v1
- Peer reviewed and published scientific report.
Goswami, Ria, Veronica S. Russell, Joshua J. Tu, Charlene Thomas, Philip Hughes, Francine Kelly, Stephanie N. Langel, et al. 2021. “Oral Hsp90 Inhibitor SNX-5422 Attenuates SARS-CoV-2 Replication and Dampens Inflammation in Airway Cells.” IScience 24 (12): 103412. https://doi.org/10.1016/j.isci.2021.103412. https://www.cell.com/iscience/fulltext/S2589-0042(21)01383-3.