Using different types of assays, researchers found both cellular and humoral immune responses for up to eight months in coronavirus disease 2019 (COVID-19) patients who recovered from mild to moderate disease.
The effect of COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) varies widely among people. Some people show no symptoms, whereas the disease is severe and even fatal in others.
Both humoral immune response, the immune response from antibodies, and cellular immune response, immunity provided by processes inside the infected cell, are believed to be important in the severity of infection. Most patients who have recovered show IgG antibodies against SARS-CoV-2 about 10 to 21 days after infection.
However, some patients who are asymptomatic or have mild disease show delayed antibody formation or no antibodies. Persistence of antibodies depends on the antibody class and infection severity. IgA and IgM antibodies drop rapidly, but IgG levels persist for several months, especially in patients who have recovered from moderate or severe disease.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Studies have shown that cellular immune response is also important. Specific T-cell responses are seen in patients with mild disease, while patients with severe disease showed a dysregulated cellular immune response.
Testing convalescent sera for immune response
In a new study published in the medRxiv* preprint server, researchers report the cellular immune response of convalescent patients in Germany, who recovered from mild or moderate infection compared to that of healthy people with no history of COVID-19.
The researchers obtained blood samples from 41 patients, 57% of who were male, with an average age of 54 years, 28 to 228 days after symptom onset. An average of nine samples were collected from each individual. They also collected blood samples from 18 healthy individuals.
The team found greater release of interferon-g (IFN-g) in convalescent blood samples stimulated with SARS-CoV-2 peptides compared to unstimulated whole blood. There was no difference in IFN-g levels in healthy samples. They found no correlation between the IFN-g release and IgG antibody levels or the time after the onset of symptoms.
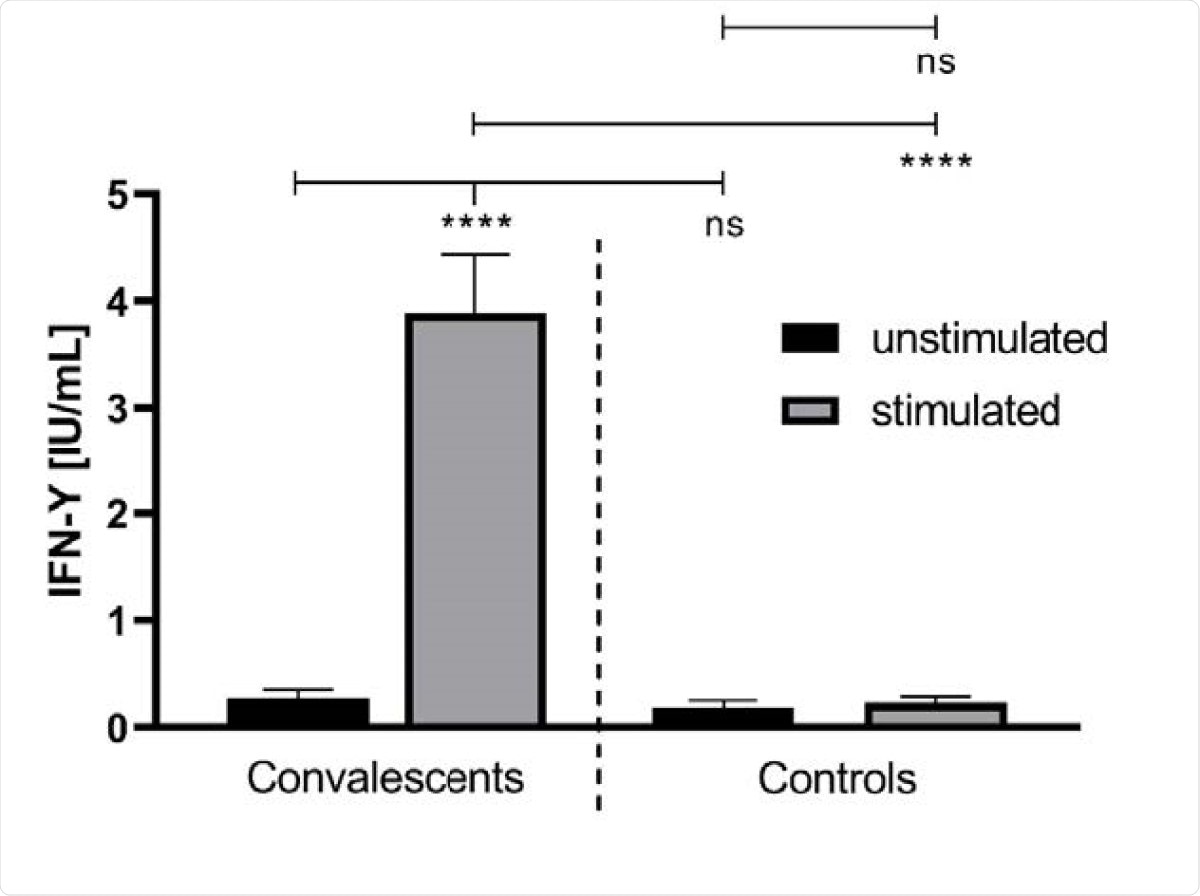
The IFN-γ concentration in unstimulated and stimulated whole blood of convalescent COVID-19 donors (n = 41) and healthy controls (n = 18). The IFN-γ release was monitored after treatment of the whole blood, donated from COVID-19 convalescents and healthy controls, with a SARS-CoV-2-specific peptide pool (grey bars). The latter contained synthetic peptides whose sequences derived from the viral spike (S), nucleocapsid (N) and membrane (M) proteins (final concentration of each peptide: 1 µg/ml). The treatment of whole blood with water served as a negative control (black bars). ****: p < 0.0001; ns: not significant (Mann Whitney U test).
Antibodies against the SARS-CoV-2 viral spike, S1 subunit, S2 subunit, and receptor-binding domain (RBD) were seen in all the convalescent samples. However, only 61% of the samples showed antibodies to the nucleocapsid protein. All controls, except one, were negative for SARS-CoV-2 antibodies.
Neutralizing antibody expression was much higher in convalescent plasma samples than samples from healthy individuals. The team also found a moderate correlation between neutralizing antibodies and general anti-SARS-CoV-2 IgG antibodies. Virus inhibition levels were higher in patients with higher neutralizing IgG antibodies than in patients with lower levels.
The authors also found increased natural killer cells in convalescent samples, in contrast to other studies that reported lower natural killer cells in patients with severe COVID-19. It is likely the increased numbers help avert a cytokine storm, known to cause severe disease.
Cellular and humoral immune response seen
The results suggest that cells retain memory of the SARS-CoV-2 virus for at least eight months after symptom onset. This corresponds to other studies that have reported specific memory B- and T-cells six to eight months after symptom onset.
IgG antibodies could be detected up to eight months in 95% of the samples. However, IgA antibodies were seen in only 20% of the samples after eight months, while 15% of the samples had no IgA even at the first sample donation. This is similar to previous reports, which observed a fast decay of this type of antibody after infection.
Using a bead-based multiplex assay, the team determined that almost all the antibodies were directed against the spike protein domains. This suggests a broad antibody spectrum, which may enable at least partial binding in viral mutations.
Antibodies to the nucleocapsid protein are found in lower number of samples compared to the spike protein. This is different from that for SARS-CoV infection, where almost all infected persons showed antibodies to the nucleocapsid protein and only about half had antibodies to the spike protein. It is possible that the nucleocapsid antibodies degrade faster, thus being undetectable with the assay used.
For one convalescent patient, no spike protein antibodies were detected, but the multiplex assay detected IFN-g after stimulation by SARS-CoV-2 peptides. This suggests cellular interferon release may be used as a test if COVID-19 is suspected, but PCR tests are negative, or if the infection has occurred quite a while ago that antibodies are no longer detectable.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources