A rapid spread of the virus that caused symptoms similar to severe pneumonia was first reported in Wuhan, China, in December 2019. Scientists found that this novel virus belonged to the family Coronaviridae and was later named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Worldwide, researchers are developing various vaccines, medicines, facemasks, and many other means to contain the SARS-CoV-2 infection.
Among various antiviral drugs, remdesivir has been approved for the treatment of coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2 infection. Remdesivir is a nucleoside analog that inhibits the SARS-CoV-2 RNA-dependent RNA-polymerase (RdRp). It is a virus polymerase inhibitor that involves the termination of both the viral transcript and newly synthesized viral genomes. However, there are some limitations that have escalated the need to develop other potential antiviral drugs that have minimal side effects and maximum efficacy.
Researchers have discovered an alternate target site of SARS-CoV-2: the 3CLpro (Mpro), main protease. This protease plays a key role in the life cycle of the virus. After the virus enters into the host cell, the positive-stranded RNA genome of the virus gets rapidly translated into two polyproteins. These polyproteins are processed into functional protein by PL2 pro and 3CLpro viral protease. Two of the main functions of 3CLpro are governing proper assembly and folding of polymerase subunits required to develop into a properly functional polymerase complex. Thereby, inhibition of 3CLpro would effectively stop the viral life cycle. Further, the unique substrate preference of 3CLpro also makes it an effective target site.
To date, PF -07304814 is the only 3CLpro inhibitor that has reached the clinical trials. It is a ketone-based covalent cysteine protease inhibitor administered as a phosphate prodrug, thereby converting to its active form, PF-90 00835231. In 2003, PF-00835231 was developed in response to the previous coronavirus epidemic as an inhibitor for the 3CLpro. However, due to the rapid decline in the infection rate, it was not brought to clinical trials, and further study about its efficacy was stopped.
Scientists believe that PF-00835231 would be effective against the novel SARS-CoV-2. This is because of a 96% similarity at the amino acid level and 100% similarity within the catalytic pocket of the 3CLpro present in both SARS-CoV and SARS-CoV-2. A recent study demonstrated the effectiveness of PF-00835231 at high micromolar levels.
In a forthcoming paper in the Journal of Virology, scientists compared the in vitro efficacy and cytotoxicity profiles of PF-00835231 and remdesivir in two human model systems for SARS-CoV-2 infection; namely, A549+ACE2 cells and polarized human airway epithelial cultures. After the initial characterization of A549+ACE2 cells to study SARS-CoV-2, an in vitro study was carried out to evaluate the efficacy and cytotoxicity of PF-00835231, GC-376 (protease inhibitor at the preclinical stage), and remdesivir in A549+ACE2 cells.
The team also conducted time-of-drug-addition assays in A549+ACE2 cells to define and compare the action time of the antiviral drugs within the SARS-CoV-2 life cycle. The role of efflux transporter Multi-Drug Resistance Protein 1 (MDR1) on the antiviral efficacy of PF-00835231 was also studied. The main focus of the study was to provide in vitro evidence of the potential PF-00835231 as an effective antiviral drug for SARS-CoV-2 and also highlight its negative effects based on prior studies.
This research has shown that both PF-00835231 and remdesivir are similarly potent in studying a model of polarized human airway epithelial cultures (HAEC). However, in A549+ACE2 cell assay, PF-00835231 revealed better activity than the preclinical GC-376 and similar or marginally effect as remdesivir.
The optimal time to start antiviral drug treatment is the first week after the onset of symptoms, i.e. when the virus replication is actively ongoing. In the case of patients who are severely affected by COVID-19, the active replication of SARS-CoV-2 can be prolonged. This study revealed that intravenous treatment of PF-00835231 would remain effective for severely infected patients. Intravenous remdesivir treatment was also found to be effective against SARS-CoV-2.
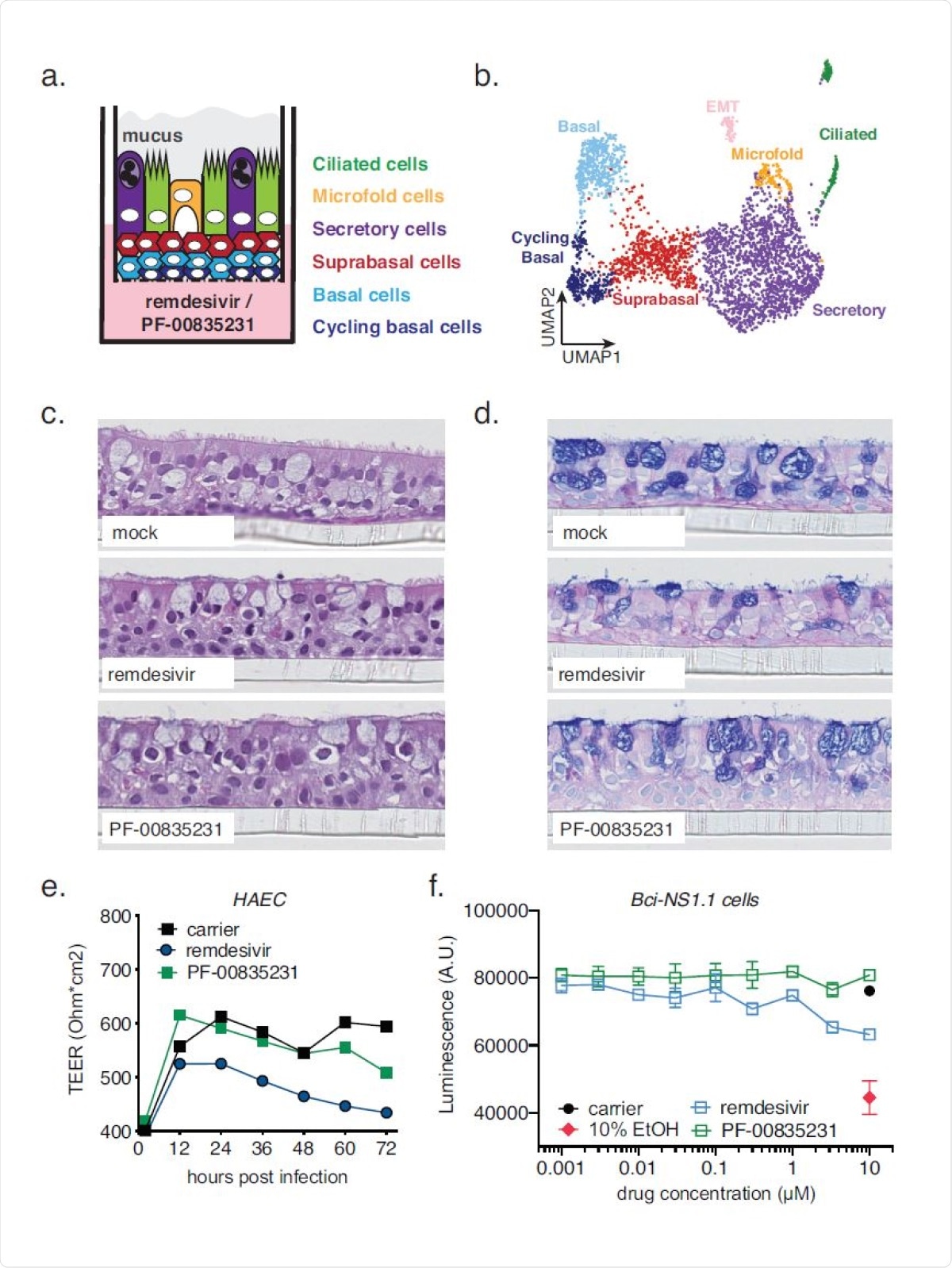
Cell composition of polarized human airway epithelial cultures (HAEC), and cytotoxicity of PF-00835231 and remdesivir. a. Schematic representation of a transwell containing a polarized HAEC in air-liquid interface. Dark blue, cycling basal cells; light blue, basal cells; red, suprabasal cells; purple, secretory cells; yellow, microfold cells; green, ciliated cells; grey, mucus. To test for cytotoxicity, drugs were added to the media in the basolateral chamber. b. Clustered UMAP of single cells determined by single-cell RNA sequencing from n=3 uninfected HAEC. Clusters were determined by markers from the literature(37, 38) and by differentially expressed marker genes for each cluster determined by Wilcox test. c., d. Representative cross-sections of uninfected HAEC, 72 h post treatment with 10 µM PF-00835231 or 10 µM remdesivir. H&E (c.) or PAS-Alcian blue staining (d.). e. Trans-epithelial resistance (TEER) in drug1205 treated, uninfected HAEC over time as a measure of epithelial integrity. Means ? SEM from n=3 independent experiments. f. CellTiter-glo assay on undifferentiated, basal-like Bci-NS1.1 precursor cells. Means ? SEM from n=3 independent experiments.
The current research has also demonstrated a significant synergistic effect between PF-00835231 and remdesivir in inhibiting SARS-CoV-2. Researchers believe that the use of multiple antiviral drugs with different modes of action or target sites would efficiently circumvent cross-resistance caused by mutations. Hence, the development of antiviral treatments using multiple antiviral drugs would significantly improve antiviral therapy in COVID -19.
To summarize, the team's research reveals the significance of the novel antiviral drug, PF–00835231, against SARS-CoV-2 with the help of 3D in vitro models of human airway epithelium. This would help to decrease the mortality rate of COVID-19 and also pave the way to explore new treatment methods for other harmful viruses.
Journal reference:
- Maren de Vries, Adil S. Mohamed, Rachel A. Prescott, Ana M. Valero Jimenez, Ludovic Desvignes, Rebecca O'Connor, Claire Steppan, Joseph C. Devlin, Ellie Ivanova, Alberto Herrera, Austin Schinlever, Paige Loose, Kelly Ruggles, Sergei B. Koralov, Annaliesa S. Anderson, Joseph Binder, Meike Dittmann. A comparative analysis of SARS-CoV-2 antivirals characterizes 3CLpro inhibitor PF-00835231 as a potential new treatment for COVID-19, Journal of Virology Feb 2021, JVI.01819-20; DOI: 10.1128/JVI.01819-20 https://jvi.asm.org/content/early/2021/02/19/JVI.01819-20