A team of international scientists has recently evaluated a surrogate virus neutralization test for severe acute respiratory coronavirus 2 (SARS-CoV-2) and observed that it is highly efficacious for detecting anti-SARS-CoV-2 neutralizing antibodies in serum samples. According to the scientists, the test can be effectively used for population-level serosurveillance and evaluation of vaccine efficacy. The study is currently available on the medRxiv* preprint server.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
As of February 28, 2021, there have been 113 million confirmed coronavirus disease 2019 (COVID-19) cases, including 2.5 million deaths, reported to the World Health Organization (WHO). However, several serological surveys have indicated that there may be many more COVID-19 cases that remained undiagnosed because of the asymptomatic nature of the disease as well as inadequate sensitivity of testing methods. Thus, testing anti-SARS-CoV-2 antibody levels in the blood is considered to be more accurate in determining the exact prevalence of COVID-19 both at national and regional levels. Moreover, antibody testing plays a pivotal role in determining the long-term efficacy of COVID-19 vaccines.
The methods typically used for serosurveillance, such as ELISA, lateral flow immunoassay, and multiplex immunoassay, mostly detect total levels of binding antibodies in the blood and thus provide information about past infection. However, these methods could not inform about the presence of neutralizing antibody levels in the blood. On the other hand, conventional virus neutralization tests (VNTs) used for detecting neutralizing antibodies require biosafety level 3 laboratories and are generally very laborious and time-consuming. To overcome these difficulties, a surrogate VNT has been recently developed and marketed by GenScript Biotech, which can qualitatively and quantitatively detect anti-SARS-CoV-2 neutralizing antibodies within 2 hours in a biosafety level 2 laboratory.
In the current study, the scientists have evaluated the efficacy of this surrogate VNT in determining anti- SARS-CoV-2 neutralizing antibodies in serum samples collected from COVID-19 patients. The test is based on the principle of ELISA wherein neutralizing ability of antibodies is measured by inhibiting the interaction between spike receptor-binding domain (RBD) and host angiotensin-converting enzyme 2 (ACE2) receptor.
Study design
For the analysis, the scientists collected 316 serum samples from hospitalized severe COVID-19 patients and healthcare workers with mild or asymptomatic COVID-19. These samples were collected from different hospitals in Belgium at different time points after COVID-19 diagnosis to ensure that the serum samples with both high and low antibody titers were included in the study. They also tested a separate set of 184 serum samples collected from healthcare workers from the Democratic Republic of Congo. These samples were suggested to be SARS-CoV-2 positive by ELISA-based antibody detection assays.
To evaluate the efficacy of the surrogate VNT, they used a Luminex multiplex immunoassay and the conventional VNT as reference methods to screen patient-derived serum samples. The sensitivity of surrogate VNT was calculated at 20% and 30% inhibition levels, which correspond to 98% and 100% specificity, respectively.
Important observations
The analysis of Belgian samples revealed that the sensitivity of surrogate VNT was 94%, which was slightly lower than the sensitivity of conventional VNT (98%) and Luminex multiplex immunoassay (96%). The scientists observed that the sensitivity of both conventional and surrogate VNTs decreased at high specificity targets. A set of 16 samples that were collected from COVID-19 patients 14 days after COVID-19 diagnosis or 5 months after infection showed positive results on conventional VNT but negative results on surrogate VNT. This indicates that the timing of sample collection may affect the sensitivity of serological assays.
Based on the analysis of African samples, the scientists estimated sensitivity of 88% for surrogate VNT, which was lower than the sensitivity of conventional VNT (99%). Using further statistical analysis, they observed a significant correlation between antibody titers of different serological assays included in the study.
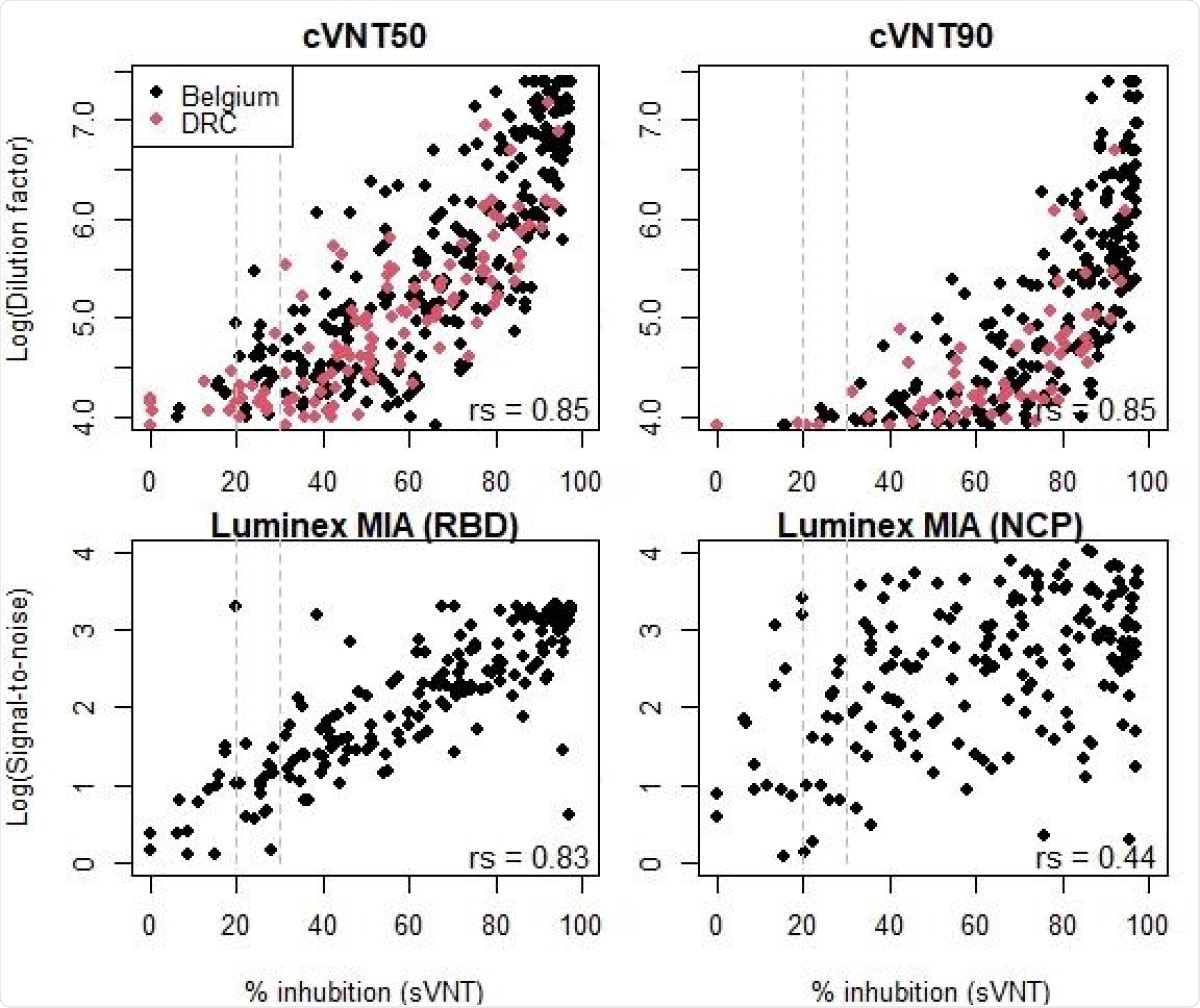
Correlations between the percentage of inhibition measured by the surrogate viral neutralisation test (sVNT) and the log(dilution factors or signal-to-noise ratio) for the conventional viral neutralization test (cVNT) or the Luminex multiplex immunological assay (MIA) as calculated by the nonparametric Spearman correlation test (rs). Seropositivity cut-off levels for the sVNT are indicated by the dashed grey lines at 20 or 30% inhibition. Negative samples on the cVNT or MIA were not included in these figures.
Study significance
Overall, the study highlights the potential of a surrogate virus neutralization test for large-scale estimation of anti-SARS-CoV-2 antibodies in serum samples. Because of the relatively lower sensitivity level, the test may not be as effective as other serological assays while diagnosing SARS-CoV-2 infection at the individual-level. However, a rapid antibody detection time and lower biosafety requirement make the test particularly effective for serosurveillance at the population level, which is important for estimating the exact prevalence of COVID-19.
The scientists also mention the usefulness of the test for vaccine evaluation, particularly in socio-economically deprived countries that lack sufficient infrastructure for conducting conventional virus neutralization tests. Because the test is species and isotype-independent, it can also be used to screen potential animal populations and detect viral spillover events.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources