Researchers found about 10% of blood monocytes in coronavirus disease 2019 (COVID-19) patients are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These monocytes then die, leading to the release of cytokines – a feature associated with severe and critical COVID-19.
In some COVID-19 patients, SARS-CoV-2 infection can lead to acute respiratory distress and multi-organ failure and death. Severe disease has been associated with increased pro-inflammatory cytokines and C-reactive protein.
Myeloid cells like monocytes, macrophages, and dendritic cells activate the sensors and receptors, called inflammasomes, which induce inflammation in response to infection. These cells are generally the most important source of inflammatory cytokines. Other pathways like NF-kB activation can also cause severe inflammation.
Researchers have found signs of inflammasomes in the blood of SARS-CoV-2 infected patients. Upon infection, they assemble into large complexes that activate the inflammatory caspase-1, which processes the cytokines interleukin-1 and pore-forming protein GSDMD that damage cell membrane, leading to cell death and inflammatory cytokine release.
But, what activates inflammasomes in the monocytes, a type of white blood cells, is a phenomenon that remains unclear. It is possible that they also become infected by SARS-CoV-2. However, monocytes do not express the angiotensin-converting enzyme 2 (ACE2), the SARS-CoV-2 receptor.
Cell death biomarkers in monocytes
In a study published in the medRxiv* preprint server, researchers reported the results of their investigation on how the virus triggers inflammation. They isolated mononuclear cells from 19 healthy donors and 22 COVID-19 patients. About 11% of the monocytes in the COVID-19 patients showed signs of membrane damage. This is a large number to detect because dying cells are difficult to detect as the body rapidly eliminates them.
The team analyzed the plasma from the samples and found high levels of specific markers for pyroptosis, a type of cell death, in the COVID-19 patients. IL-6, TNFa, several growth factors, and chemokines were also high in COVID-19 patients. In addition, the team found higher levels of pyroptosis markers in patients with severe disease compared to those with mild disease.
Immune gene analysis of 4336 severe cases compared with 623,902 control population samples revealed the GSDMD expression quantitative loci were highly associated with respiratory failure. Two other inflammasome genes, NLRP3 and NLRC4 were also associated with severe COVID-19.
Tests also revealed that a large proportion of freshly isolated monocytes in COVID-19 patients had higher levels of inflammasome adapters activated caspace-1 and ASC.
These results suggest circulating monocytes in COVID-19 patients may die of pyroptosis, releasing inflammatory cytokines and causing a cytokine storm resulting in poor clinical outcomes.
About 10% of the monocytes in COVID-19 patients also showed the presence of SARS-CoV-2 nucleocapsid protein and dsDNA, while these were not seen in the blood of healthy donors. Hence, a small portion of the circulating monocytes in COVID-19 patients shows replicating the SARS-CoV-2 virus.
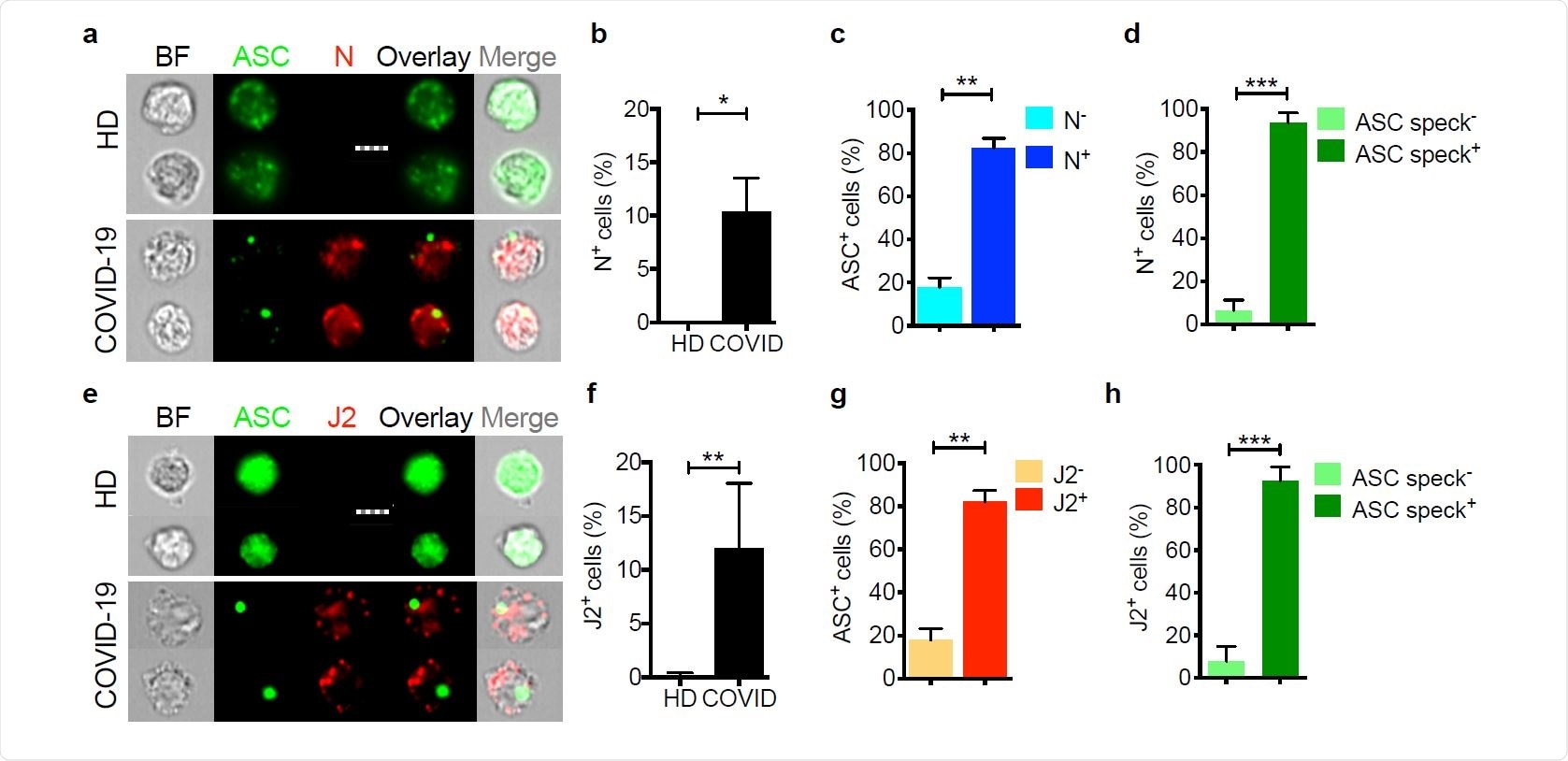
Circulating monocytes are infected with SARS-CoV-2 Circulating monocytes from HD and COVID-19 patients were purified and stained for SARS-CoV-2 nucleocapsid (N) (n=5) (a-d) or dsRNA (J2 antibody) (n=4) (e-h) and ASC. Shown are representative imaging flow cytometry images (a,e), quantification of percentage of cells that were infected by N (b) or J2 (f) staining, percentage of uninfected or infected cells (by J2 or N staining) that showed ASC specks (c, g) or percentage of cells with or without ASC specks that showed N (d) or J2 (h) staining. Scale bar, 7 µm. BF, brightfield. Mean ± S.E.M. is shown. *p<0.05, **p<0.01, ***p<0.001 by nonparametric unpaired t test (Mann-Whitney or Kolmogorov-Smirnov).
Monocyte infection triggers inflammation
Monocyte infection and cell death have not been widely seen before. This may be because many studies use frozen and thawed cells, and dying cells do not survive this process. In addition, since it was believed that monocytes do not express ACE2, researchers have not looked closely at whether they may become infected.
The results also suggest opsonizing antibodies may play a role in monocyte infection and inflammation. Studies have seen clinical deterioration coinciding with detection of antibody response against the virus, and antibody-dependent enhancement may be associated with severe disease. Characterizing the antibodies that are most effective in monocyte infection will be important in identifying monoclonal antibodies for the treatment and identifying if vaccines generate antibodies that cause monocyte infection and inflammation.
The researchers also found a one-to-one relation between monocyte infection and caspase-1 activation. However, it is not clear how SARS-CoV-2 infection activated inflammasomes. Some coronaviruses are known to express three ion channel proteins that disrupt ion concentrations and activate NLRP3 and could be a likely mechanism.
The observation of plasma biomarkers of pyroptosis in COVID-19 patients and their correlation to severe disease could potentially be used as a diagnostic test for identifying who might develop severe disease. Testing approved drugs that inhibit inflammatory cytokines or GSDMD could also be worth investigating.
In addition, future studies could also look at other infected cells that express GSDMD, as sources of inflammation, such as pneumocytes and macrophages in the lung.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Junqueira, C. et al. (2021) SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release. medRxiv. https://doi.org/10.1101/2021.03.06.21252796, https://www.medrxiv.org/content/10.1101/2021.03.06.21252796v1
- Peer reviewed and published scientific report.
Junqueira, Caroline, Ângela Crespo, Shahin Ranjbar, Luna B. de Lacerda, Mercedes Lewandrowski, Jacob Ingber, Blair Parry, et al. 2022. “FcγR-Mediated SARS-CoV-2 Infection of Monocytes Activates Inflammation.” Nature, April, 1–13. https://doi.org/10.1038/s41586-022-04702-4. https://www.nature.com/articles/s41586-022-04702-4.