For most of 2020, the coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), severely impacted economic and social activity in a diverse range of fields. As vaccines are being rolled out in many countries across the world, a decline in adherence to social distancing and face mask usage has been observed. The issue of how to relax non-pharmaceutical interventions (NPIs) such as these safely is still unresolved, however.
The current scenario
Within the USA, COVID-19 deaths have crossed the 500,000 mark. Since the emergence of the pandemic, NPIs were in use in most parts of the country, although in a somewhat patchy way.
However, social distancing, as well as business and school closures, have high social costs and severe economic fallout. Their unsustainability has led to increased pressure on the governments to develop vaccines that can allow their withdrawal.
The relaxation of NPIs has always been followed by higher community transmission, with subsequent waves of COVID-19 cases in the fall and winter. This vicious cycle has led to the repeated re-implementation of NPIs.
With the emergency use authorization of three COVID-19 vaccines so far, things are looking up. The shortage of vaccines at present is a potential roadblock, but the USA has commitments from manufacturers which should overcome this obstacle. Nonetheless, the present goal is to vaccinate as many people as possible with one dose at least.
This strategy is based on empirical thinking since the baseline transmission, the vaccine efficacy at one and two doses, respectively, and the mechanism of action of the vaccine are all unclear at this stage for this infection.
Vaccines in use at present
The indirect effects of vaccination are just as important as the direct effects when it comes to protecting the individual against SARS-CoV-2 infection, since they prevent virus spread. Unfortunately, there is little data on this as yet, as most vaccine efficacy reports have centered on protection against symptomatic infection – understandably so in view of the urgent need to reduce COVID-19 deaths and healthcare burden.
The available vaccines in the USA are the two mRNA vaccines from Pfizer/BioNTech and Moderna, with two doses reported to have protective efficacy of 94% or higher against symptomatic SARS-CoV-2 infection. The most recently approved Johnson and Johnson vaccine is a single dose formulation with 66% protection against symptomatic infection and 85% against severe disease.
The high vaccine efficacies indicate that some protection against transmission is likely. The reduced chances of infection in vaccinated individuals indicate that NPIs can be safely relaxed for this group, and especially for younger people in whom the risk of severe COVID-19 is already low.
Indeed, vaccinated individuals do tend to increase their level of social interaction soon afterward, and the Centers for Disease Control and Prevention (CDC) has issued its advisory on safe activities for fully vaccinated people.
What are the results?
The current model deals with preferred NPI relaxation models for unvaccinated vs. vaccinated people regarding the population risk for infection.
The study shows that vaccination benefits result from both the timeline of NPI relaxation and the speed of vaccine rollout. Too early relaxation of NPIs, as has occurred in many states already, before a large fraction of the population is immunized, could push up the rate of deaths and admissions significantly.
NPIs complement vaccination
For instance, if NPIs are immediately relaxed to pre-pandemic levels within 30 days in an unvaccinated population, the deaths could shoot up by an additional 1.2 million. If vaccination is used, protecting against infections but not against disease progression once infected, this level of NPI relaxation would add 1 million more deaths.
A delay in reopening by 3 months with a vaccine rollout of 3 million doses a day, with the same vaccine performance as above, followed by rapid NPI relaxation, would reduce deaths to approximately 84,000 and eliminate the need for social distancing within this period.
Interestingly, this does not exceed the number of deaths with only NPIs for the duration of the outbreak, indicating the acceptability of reopening within three months of a rapid vaccine rollout.
If only NPIs are used, but not vaccination, the same period would see 1.1 million deaths because of the delayed acquisition of population immunity in this case. The use of vaccines is predicted in this model to reduce the incidence to almost zero in three months if a rapid pace of vaccination is maintained.
With high efficacy, a one-dose strategy could achieve safe relaxation even earlier, but not if the efficacy is less than 80%. With a rapid rollout, this risk is not worth taking, as a 3-month delay will reduce the risk to almost nothing.
NPIs relaxation based on vaccination rollout
The delay in reopening allows adequate vaccine overage. However, if those who are fully vaccinated are allowed to resume normal social interactions with each other, there is no marked change in the number of deaths. The risk is somewhat higher if the one-dose strategy is adopted and if the vaccine is not as protective against asymptomatic infection.
When it comes to unvaccinated and older individuals, safe reopening dates depend on the speed of rollout and the dosing strategy. With two doses, and if 3 million doses are given each day, the optimum delay in reopening is three months. The one-dose strategy loses its charm in this case.
Within these 3 months, at this pace, about 40% of the population will be vaccinated, which in combination with natural immunity will be sufficient to achieve herd immunity.
At only 1 million doses a day, the outbreak will continue to take its course, and NPIs cannot be relaxed, for 7 months from the beginning of the vaccination campaign.
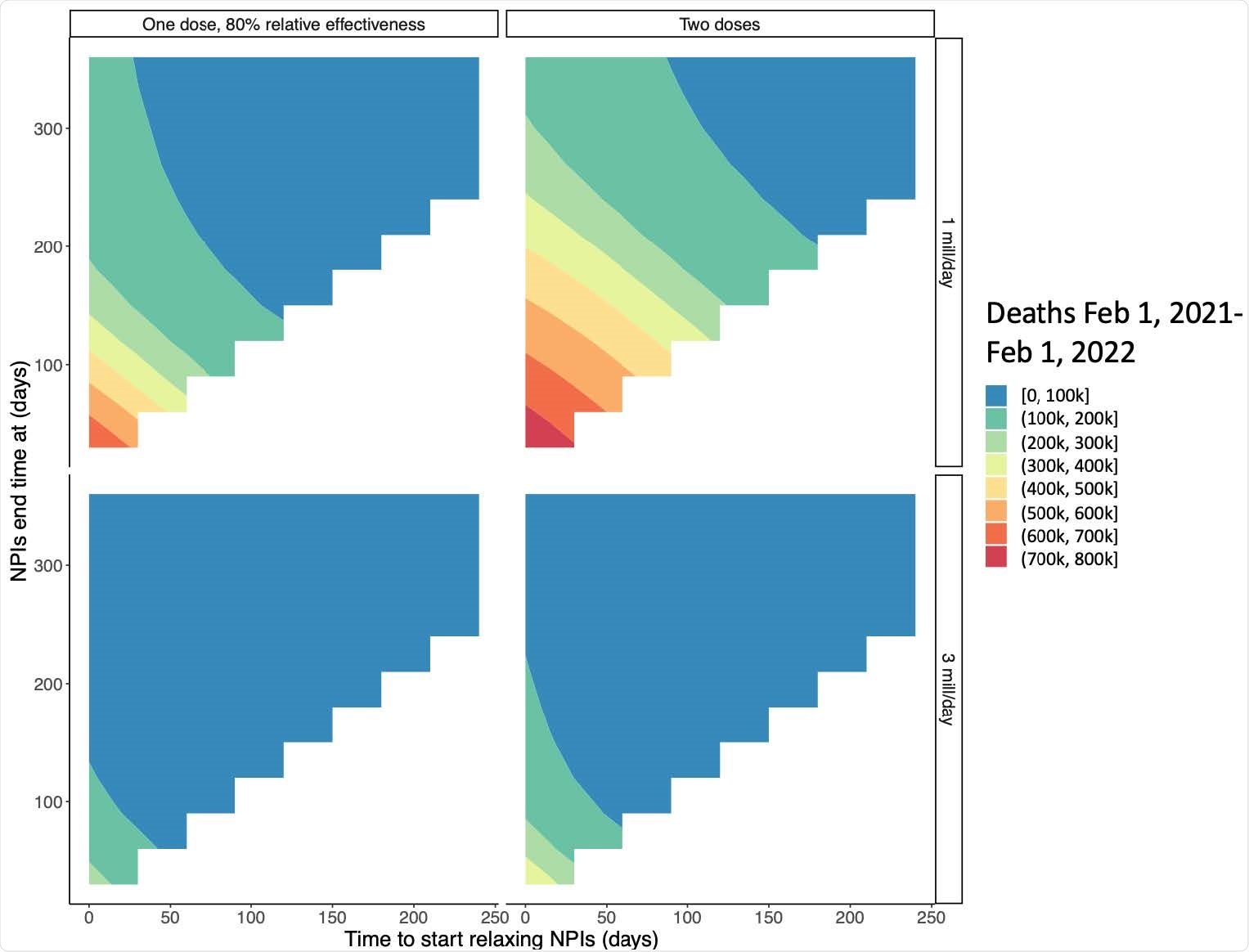
Interaction between time to regain pre-pandemic social interactions and the start time of relaxation by vaccine strategy and rollout speed. The x-axis shows the number of days between February 1, 2021 and the start of further NPI relaxation and the y-axis shows when normal interactions are restored after reopening begins (corresponding to the speed of relaxation). Colors show expected deaths for each reopening strategy. For these simulations, vaccinated individuals are assumed to begin relaxing immediately and a susceptibility only vaccine is modeled.
Effects on healthcare systems
The 3-month delay coupled with a rapid rollout of vaccines is also beneficial in relieving the load on the healthcare workers and the hospital system. A repeated peak of hospitalizations may be expected if relaxations occur too early or if vaccine rollout is too slow.
This may also be the case if only one dose is used or if the vaccine is protective against symptomatic disease more than transmission. Even with two doses, the peak of hospitalization would be higher than at the start of 2021.
If the delay can be extended to more than 3 months, a more rapid reopening can succeed without a second wave of cases.
Effect of baseline immunity
The model also shows the importance of baseline immunity in the population. If it is lower than expected, a two-dose vaccine rolled out at top speed (3 million doses a day) with continued NPIs for three months would reduce deaths to less than 1,78,000. Another two months of delay would reduce the deaths by 62,000.
If NPIs are relaxed within three months, in the same scenario, over 3,10,000 deaths may be expected. With a slow rollout, NPIs will need to be extended by seven months.
What are the implications?
The vaccination program can accelerate the return of normal social and economic interactions to pre-pandemic levels. However, the pace of vaccination and of NPI relaxation plays a crucial role in determining how many deaths will occur over the coming year.
Without vaccination and complete reopening, over a million deaths are likely over this year, with high community spread. At the other extreme, two doses of vaccine with a three-month delay in NPI relaxation can reduce deaths by 93%, with the end of the outbreak being predicted in three months.
The importance of rapid vaccine rollout is that it alone can shorten the epidemic. With a slow rollout, even with a delay in reopening for three months, and with a step-wise reopening, the virus will continue to spread over the coming year.
The benefits of vaccination are enhanced greatly by a delayed relaxation of NPIs. Not only does vaccine coverage improve, but treatments may be developed to reduce mortality in the interim.
A significant conclusion is that vaccinated individuals can relax social distancing with each other without undue risk to the population at large. This could help a large percentage of the population to return to normal activities more quickly, and thus reduce the costs of continued NPIs for the rest of the community.
Since the prioritized vaccination campaign targets healthcare workers, who interact with a larger number of people, the actual reduction in risk if they are allowed to relax NPIs earlier could possibly be a lot larger than envisaged by this model.
Evidence on the role of vaccines in reducing infectiousness is awaited, though early results are promising. Further studies should address the role of new variants of the virus which are resistant to these vaccines, and/or which are more transmissible. These could make the need for NPIs to be continued longer than modeled in the current study.
The one-dose strategy may promote immune escape, but the evidence is still awaited for this claim. Finally, the role of waning immunity was not addressed in this model, though it may play a key role in determining the need for follow-up booster doses in the future.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.