A brief report by researchers at the University of Virginia, USA, proposes using an antibody-based treatment with a plug-and-play strategy to inhibit the ability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to invade human cells.
The treatment results show it works by blocking the host protease-activation function of the spike protein — one of the first steps needed for viral entry.
The researchers write:
Our study reveals analytical, safe, and selective mechanistic insights for SARS-CoV-2 therapeutic design and is broadly applicable to the future coronaviridae family members (including mutant variants) exploiting the host protease system for cellular entry.”
The study “An Innovative antibody-based Plug-and-Play strategy for SARS-CoV-2” is available as a preprint on the bioRxiv* server, while the article undergoes peer review.
How they did it
The team hypothesized that they could prevent viral fusion into host cells by targeting both the SARS-CoV-2 virus and furin near the viral infection site. They engineered a 22-IgG1 Fc — a spike protein receptor binding domain-targeting antibody. The researchers incorporated furin substrate sites in the Fc-extended linker region to compete with cellular furin protease function and prevent viral entry. The approach was named FuG1 (Furin-IgG1).
This method could be used alone for the above purpose and used to co-target human lung cell antigens and the spike protein.
Study findings
Given that 293-ACE2 cells could have folate receptor levels similar to those observed in human cancer cells, the team added an anti-FOLR1 farletuzumab in the FuG1 strategy. After two hours of 293-ACE2 cells being exposed and subsequently infected with the coronavirus spike protein, farletuzumab-FuG1, and 22-FuG1 antibodies were added.
Their results showed the FuG1 antibodies helped prevent syncytia formation.
As expected, FuG1 inhibited spike conversion into S2 in a dose dependent manner. GAPDH revival in lysates indicate reversal of cytopathic cellular state. When tested using spike pseudovirus, 22-FuG1 also inhibited proteolytic activation of spike to generate S2 upon addition on cells,” wrote the research team.
The antibodies work in different ways. 22-FuG1 works by targeting furin residues after a delayed spike expression. Meanwhile, the farletuzumab-FuG1 saturates furin by occupying the space of FOLR1+ cells. The researchers confirmed its function and specificity with infected cells by treating spike-infected cell cultures with 50µg 22-IgG1, farletuzumab-FuG1, and 22-FuG1.
Their results showed that farletuzumab-FuG1 showed a 30% risk reduction of developing syncytia. The 22-FuG1 antibodies displayed approximately a 90% risk reduction and partially reduced S2 conversion in lysates.
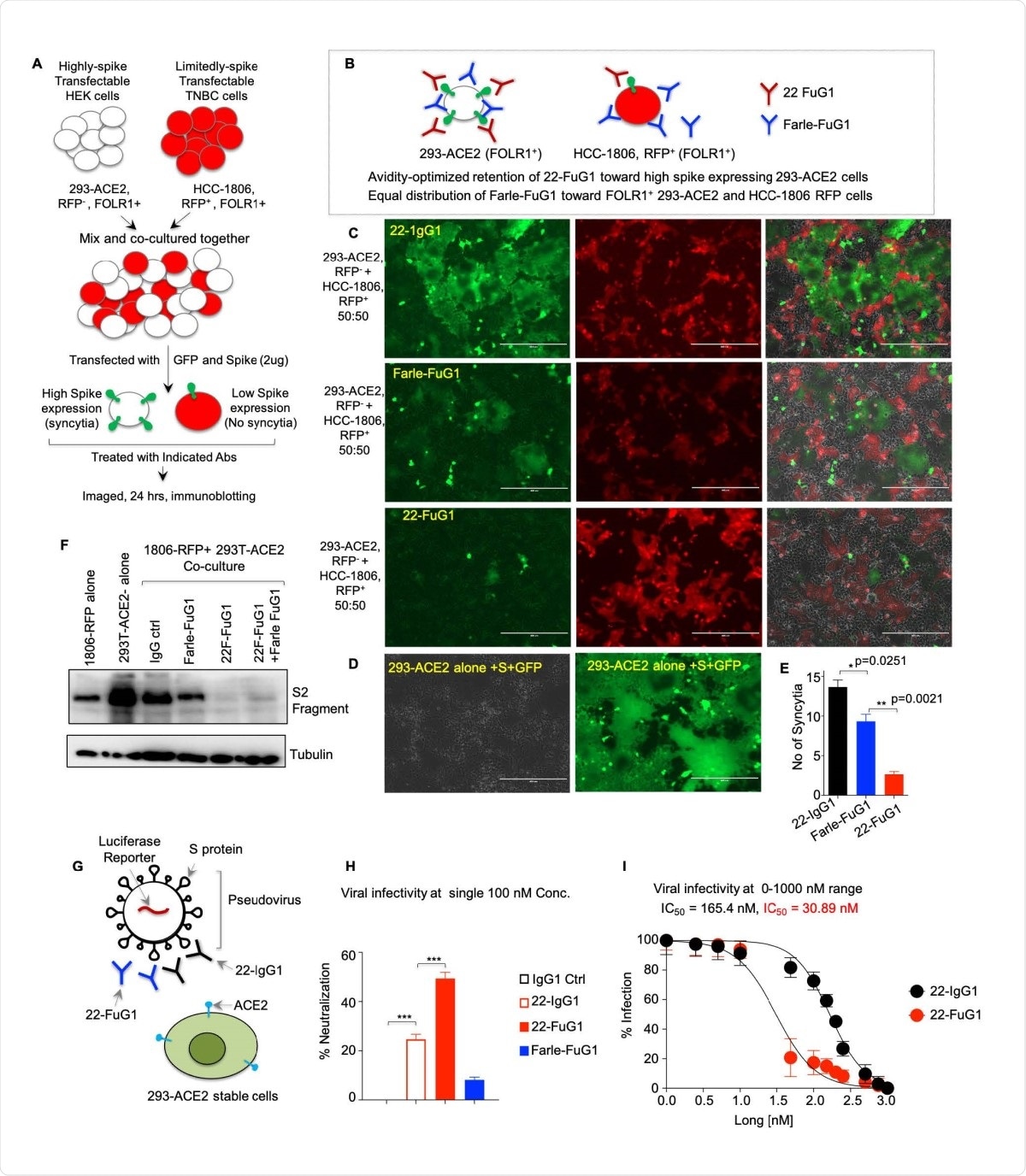
FuG1 antibody is selective in blocking SARS-CoV-2 entry. (A) Schematic of the co-culture experiment described in D and E. (B) Schematic showing preferred binding of 22- FuG1 (Red) to highly expressed spike on 293-ACE2 cells, while farletuzumab-FuG1 (Blue) distributes equally against both cell lines. (C-D) Co-cultured cells treated with 22-IgG1, Farle- FuG1 and 22-FuG1 antibodies. (E) Number of syncytia from C (n=3). (F) Total lysates from spike-transfected: HCC1806 cells, 293-ACE2 cells alone, and cocultured-cells treated with indicated antibodies were immunoblotted for S2. (G) Schematic of the experiment described in H, I. (H-I) Pseudoviral neutralization assays after treatment of Farle-FuG1 and 22-FuG1 antibodies at increasing concentration. IC50 values are shown (n=3).
Compared to using IgG1 antibodies, pseudovirus neutralization assays showed the 22-FuG1 antibody strategy was significantly more effective in having specificity against spike-infected cells. The 22-FuG1 antibodies showed a greater than 6-fold neutralizing ability against the spike protein.
The researchers suggest FuG1 antibodies work to prevent viral entry by interfering with the relationship between ACE2 expression and the receptor-binding domain (RBD) of the spike protein.
The described FuG1 strategy gives spike target mediated specificity over described furin and TMPRSS2 small molecule inhibitors. Furthermore, additional antibody dual-specificity based FuG1 co-targeting lung enriched receptor could be easily engineered on the described foundation for high specificity. As epitope of CR3022 does not fully overlap with ACE2-RBD binding, FuG1 antibodies that strictly interfere with ACE2-RBD function are expected to be highly effective,” concluded the research team.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.