Although several strategies have been implemented to combat the COVID-19 pandemic, it continues unabated. Despite several vaccines being approved and used, vaccination rates have been slow, and there have been challenges in the equitable distribution of vaccines. These, along with decreasing immunity in persons already infected and the emergence of new virus variants, make it difficult to contain the pandemic.
Several therapeutic strategies have used convalescent sera and human monoclonal antibodies. However, new variants have emerged, with mutations that can evade these therapeutics.
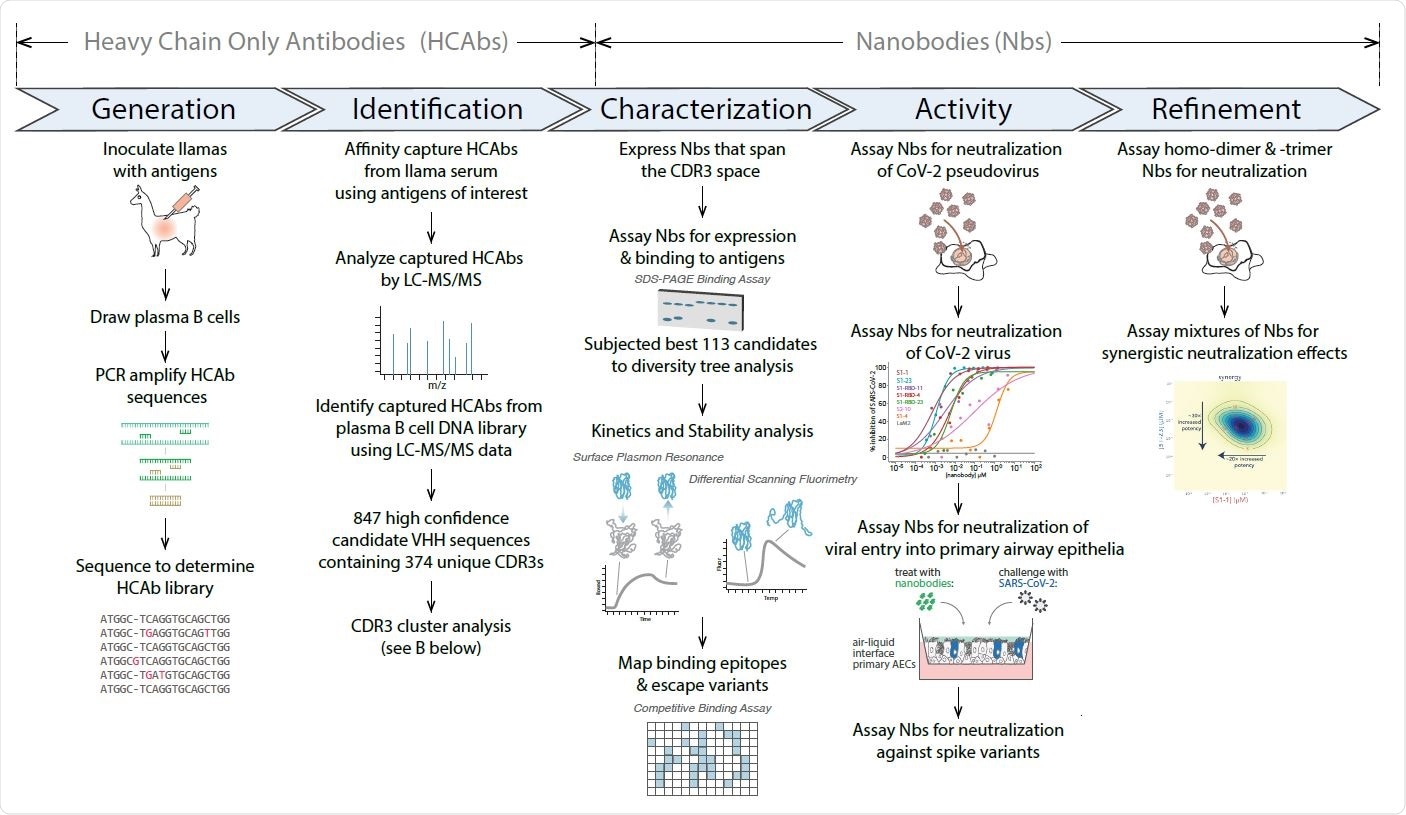
An alternative to monoclonal antibodies is nanobodies. These are smaller proteins derived from animals like llamas and alpacas. Their small size allows them to bind to regions that are generally not accessible to the larger monoclonal or polyclonal antibodies. They are also simpler to manufacture as they can be easily cloned and expressed in bacteria. They can also be delivered directly to the lungs via nebulization.
However, the nanobodies also recognize regions of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein receptor-binding domain (RBD), which can cause escape mutations, reducing their potency.
Pre-screening protocol to select llamas with naturally strong immune responses, as determined by activity against standard animal vaccines

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Testing nanobody neutralization capability
In a study published in the bioRxiv* preprint server, researchers report a collection of a large number of nanobodies that could potentially be resistant to escape mutations.
To build their suite of nanobodies, the team refined and optimized their previous method. They chose llamas with a strong immune response to SARS-CoV-2 and selected 113 nanobodies for further testing. A large proportion of the antibodies bind to the RBD, while the other bind to the non-RBD portion of the S1 subunit of the spike protein and the rest to the S2 subunit.
To identify nanobodies that will be resistant to virus mutations, the team selected a large number of high RBD binding nanobodies and tested them against the B.1.1.7 variant. Of the seven nanobodies they tested, they found six showed strong binding to the variants. In addition, they also chose nanobodies that bind to the non-RBD regions of the spike protein.
The authors then categorized the antibodies depending on what parts of the RBD they bind to. Their analysis revealed the RBD-binding nanobodies could be classified into three distinct groups. Within each group, the nanobodies could be grouped into bins so that some bind to distinct epitopes and partially overlap, binding to other separate epitopes.
This suggests two or more nanobodies can bind to the RBD at the same time. Further analysis revealed at least three different nanobodies could bind to the RBD simultaneously, which will be important in designing nanobody cocktails for therapies.
The nanobodies were potent in neutralizing SARS-CoV-2 pseudoviruses, with 16 nanobodies neutralizing the pseudovirus at less than 20 nM concentration. Nanobodies that target the non-RBD regions and the S2 subunit also neutralized the virus but at higher concentrations. The authors write this is the first evidence of nanobody neutralization targeting regions outside the RBD.
Nanobody cocktails more potent against escape variants
The team also tested the best nanobodies against pseudoviruses carrying the spike protein of the B.1.351 variant. Some nanobodies showed no neutralization activity against this variant, while one showed similar neutralization as the wild-type virus. Two nanobodies, however, showed increased neutralization activity against the variant. The nanobodies that neutralized the pseudovirus also neutralized the real SARS-CoV-2 virus and human airway epithelial cells. The team also found some nanobody combinations can work synergistically and increase potency dramatically.
Using nanobody cocktails mixed with the SARS-CoV-2 virus and allowing multiple replications, the authors also identified mutations that resist neutralization. Some potent nanobodies caused mutations at the same location as generated by antibodies in human convalescent sera, such as E484K, indicating the ACE2 binding site is a point of vulnerability for neutralization.
However, nanobody cocktails also increased the genetic barrier for escape. Mixing two nanobodies required the virus to undergo two different amino acid substitutions, making it more difficult for it to escape neutralization. Carefully choosing mixtures with more nanobodies may further decrease escape mutations.
Thus, the escape experiments show that currently used or newer therapeutics may yet lose their potency with newer virus variants emerging, which is very likely as some of the mutations seen in the experiments have not been seen in human isolates so far. However, using a combination of nanobodies, which can work synergistically to improve neutralization potency, the large collection of nanobodies generated by the team may help develop more potent treatments.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Mast, F. D. et al. (2021) Nanobody Repertoires for Exposing Vulnerabilities of SARS-CoV-2. bioRxiv, https://doi.org/10.1101/2021.04.08.438911, https://www.biorxiv.org/content/10.1101/2021.04.08.438911v1
- Peer reviewed and published scientific report.
Mast, Fred D, Peter C Fridy, Natalia E Ketaren, Junjie Wang, Erica Y Jacobs, Jean Paul Olivier, Tanmoy Sanyal, et al. 2021. “Highly Synergistic Combinations of Nanobodies That Target SARS-CoV-2 and Are Resistant to Escape.” ELife 10 (December). https://doi.org/10.7554/elife.73027. https://elifesciences.org/articles/73027.