A study recently published in the journal Matter has demonstrated the development and validation of a nanoparticle-based formulation, capable of completely inhibiting the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection blocking the interaction between viral spike protein and host cell angiotensin-converting enzyme 2 (ACE2).
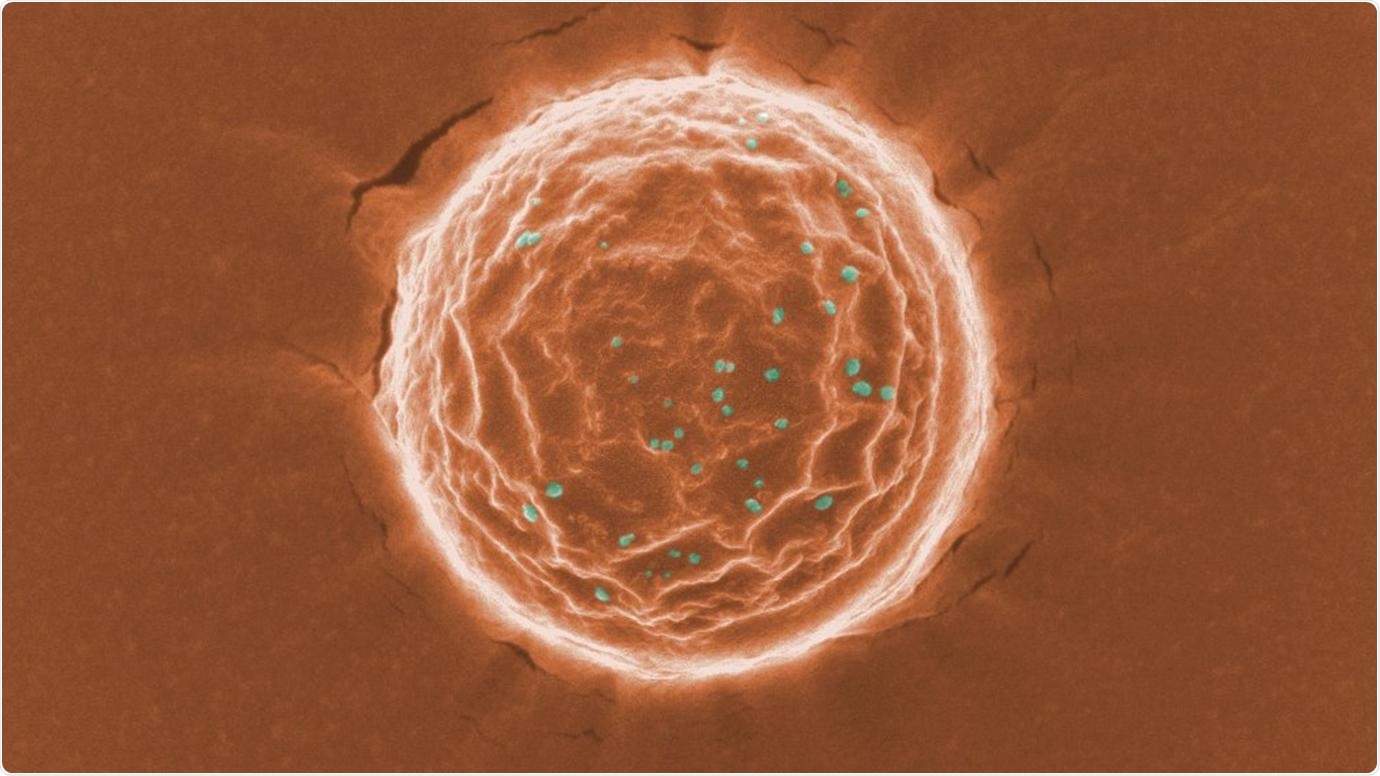
A scanning electron microscope image of a nanotrap (orange) binding a simulated SARS-CoV-2 virus (dots in green). Scientists at the University of Chicago created these nanoparticles as a potential treatment for COVID-19. Image courtesy Chen and Rosenberg et al.
Background
SARS-CoV-2, the causative pathogen of coronavirus disease 2019 (COVID-19), is an enveloped, single-stranded, positive-sense RNA virus belonging to the human Coronaviridae family. It is now well-established that SARS-CoV-2 infection initiates with the binding of viral spike protein to host cell ACE2 receptor. Therapeutic interventions, such as soluble recombinant ACE2 and anti-SARS-CoV-2 neutralizing antibodies, targeting the spike-ACE2 interaction, have been shown to block the viral entry and prevent infection effectively. For the successful delivery of these therapeutics, nanoparticle-based formulations have shown significant efficacy.
In the current study, the scientists have designed and developed a nanoparticle-based formulation termed “Nanotrap” to inhibit and eliminate SARS-CoV-2.
Development of Nanotrap
The Nanotraps of different diameters (200, 500, and 1200 nm) were designed with biodegradable polylactic acid polymeric core and liposome shell materials. The polylactic acid core was used to ensure mechanical stability and increase the surface area of the Nanotrap. The liposome shell materials covering the core structure were used to mimic the natural functions of a cell membrane.
The Nanotrap surface was functionalized with either recombinant ACE2 proteins or anti-SARS-CoV-2 neutralizing antibodies. A high density of these proteins was maintained on the surface to ensure high-efficiency binding with the virus (high avidity). The small size, high avidity, and high diffusivity of the Nanotrap make it highly efficient to inhibit SARS-CoV-2 infection.
Furthermore, the Nanotrap surface was decorated with phosphatidylserine ligands to ensure viral clearance through macrophage-mediated phagocytosis. Phosphatidylserine ligands present on the cell membrane are known to facilitate macrophages to recognize and engulf apoptotic cells for phagocytosis.
Important observations
Initially, the scientists conducted a series of experiments to examine the structural and functional integrity of Nanotraps. The findings revealed that Nanotraps effectively captured pseudotyped SARS-CoV-2 and undergone macrophage-mediated phagocytosis. Importantly, they observed that the Nanotraps interacted with the virus first, followed by macrophage-mediated phagocytosis. Of all formulations, the Nanotraps with 500 nm diameter showed the highest antiviral efficacy.
In vitro SARS-CoV-2 neutralization
By incubating ACE2-bound and antibody-bound Nanotraps with SARS-CoV-2 spike pseudotyped lentivirus, the scientists observed that both formulations were highly effective in inhibiting SARS-CoV-2 from infecting ACE2-expressing cells. Interestingly, ACE2-bound Nanotraps were found to be more effective than antibody-bound Nanotraps in inhibiting viral infection. The scientists mentioned that lower surface density and random orientation of antibodies might be responsible for such lower efficiency.
To examine macrophage-mediated phagocytosis of Nanotraps, the scientists first checked whether macrophages are susceptible to SARS-CoV-2 infection. By incubating macrophages with SARS-CoV-2, they confirmed that macrophages do not get infected by the virus.
In the next step, by incubating ACE2-bound Nanotrap with coculture of human epithelial cells, macrophages, and pseudotyped SARS-CoV-2 particles, they observed complete inhibition of viral infection by the Nanotrap formulation. They also observed that the Nanotraps were incorporated into macrophages but not into epithelial cells. These observations indicate macrophage-specific phagocytosis of Nanotraps.
Biosafety profile of Nanotrap
Using different human cell lines, the scientists conducted a cytotoxicity assay and observed no significant cytotoxic effects of the Nanotraps. By conducting a separate set of in vivo experiments, they confirmed that Nanotraps could be successfully delivered to the mice lungs via intratracheal injection. Moreover, by conducting histological staining of vital organs and biochemical measurements, they revealed that Nanotraps did not cause any alteration in tissue anatomy, blood cell count, blood glucose level, electrolyte balance, and kidney and liver functions.
Therapeutic efficacy of Nanotraps
To determine the therapeutic potency of Nanotraps, the scientists used ex vivo perfused and ventilated healthy, non-transplantable human donor lungs. By injecting the lungs with SARS-CoV-2 pseudovirus and antibody-bound Nanotraps, they observed that Nanotraps were able to inhibit viral infection in the lung tissues completely. Finally, using Vero E6 cells, they confirmed that antibody-bound Nanotraps not only inhibited the infection with authentic SARS-CoV-2 but also outperformed the SARS-CoV-2-inhibiting efficacy of soluble therapeutic antibodies.
Study significance
The study describes the development and validation of a nanoparticle-based formulation termed Nanotrap, which exhibits high potency in abolishing SARS-CoV-2 infection in both in vitro and in vivo setups. The main advantage of the Nanotrap is that it not only inhibits SARS-CoV-2 infection but also eliminates the virus via macrophage-mediated phagocytosis.