A team of scientists from the USA and Canada has recently identified and characterized a novel small-molecule compound inhibitor of cellular serine protease TMPRSS2, which shows significant antiviral potency against emerging variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The study by researchers from the University of British Columbia, Cornell University College of Veterinary Medicine, and Université de Sherbrooke is currently available on the bioRxiv* preprint server.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
As of today, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative pathogen of coronavirus disease 2019 (COVID-19), has infected more than 154 million people and claimed 3.23 million lives globally. The approval and widespread distribution of several highly effective vaccines, along with other public health measures, now allows the possibility of controlling the COVID-19 pandemic. However, novel genetic variants of SARS-CoV-2 are emerging and spreading at an alarming rate. Notably, vaccine effectiveness may be reduced against a number of these variants, termed variants of concern (VOCs).
Besides prophylactic vaccines, severe antiviral medicines have also been repurposed as therapeutic interventions against COVID-19. One such example is remdesivir, which is a direct-acting antiviral medicine targeting RNA-dependent RNA polymerase of SARS-CoV-2. In addition, some host-directed antiviral medicines are under investigation. Host-directed medicines are expected to have better efficacy against SARS-CoV-2 because they directly target host genes, and compared to viral genes, host genes have a lower propensity for mutation.
In the current study, the scientists have identified and characterized a small molecule inhibitor targeting the human type-II transmembrane serine protease TTMPRSS2, which plays an essential role in proteolytically cleaving and priming the spike protein of SARS-CoV-2. They tested the antiviral efficacy of the inhibitor against SARS-CoV-2 and two other VOCs (B.1.1.7 and B.1.351). In addition, they have examined whether this inhibitor can reduce SARS-CoV-2-induced morbidity and mortality in mice.
Important observations
The scientists developed a library of peptide-mimicking compounds with serine-trapping warheads (ketobenzothiazole) and screened them to identify compounds capable of inhibiting the proteolytic activity of TMPRSS2. For the screening, they expressed full-length, wild-type TMPRSS2 in cells and checked whether these compounds can inhibit TMPRSS2 from proteolytically cleaving a TMPRSS2-preferred fluorogenic substrate. According to the screening findings, the compound N-0385 showed the highest potency (83%) in inhibiting TMPRSS2 at nanomolar concentrations. Notably, the compound did not show any cytotoxic effect even when used at a higher than effective concentration.
By replacing the ketone group of N-0385 with an alcohol group, the scientists proved that the integrity of the serine-trapping warhead is essential for maintaining the TMPRSS2-inhibiting efficacy of the compound.
To determine the selectivity of N-0385, the scientists conducted a series of experiments, which revealed that the Ser441 residue in the catalytic subunit of TMPRSS2 covalently interacts with the warhead ketone of the compound, causing a tight-binding mode of inhibition.
By conducting fluorescence imaging on N-085-pretreated, SARS-CoV-2-infected human lung cells and donor-derived human colonoids, the scientists measured that the compound is 99% effective in inhibiting viral translation and replication at nanomolar concentrations.
Next, the scientists determined the broad-spectrum antiviral potency of N-085 against the UK (B.1.1.7) and South African (B.1.351) variants of SARS-CoV-2. Using a previously circulating Canadian variant of SARS-CoV-2 as a reference variant, they observed that the compound has comparable efficacy in inhibiting the reference variant and the UK variant. However, they detected that the dose of N-085 required to inhibit the South African variant is relatively higher than that required for the other two variants.
Finally, the scientists conducted a set of in vivo experiments using mice expressing human angiotensin-converting enzyme 2 (ACE2) to determine the efficacy of N-085 in preventing SARS-CoV-2-induced morbidity and mortality. By intranasally treating SARS-CoV-2-infected mice with the compound once daily for eight days, they observed a significant improvement in weight reduction and survival rate. Moreover, they noticed that compared to untreated mice, the mice treated with N-085 exhibit only mild histopathological changes in the lung and brain tissues and significantly lower the amount of viral antigens in the lung and brain.
By treating the mice only for two days post-infection, they noticed 100% survival and an average of 2% weight gain, indicating that intranasal treatment with N-085 at the early infection phase can significantly reduce SARS-CoV-2-related morbidity and mortality and improve clinical outcomes.
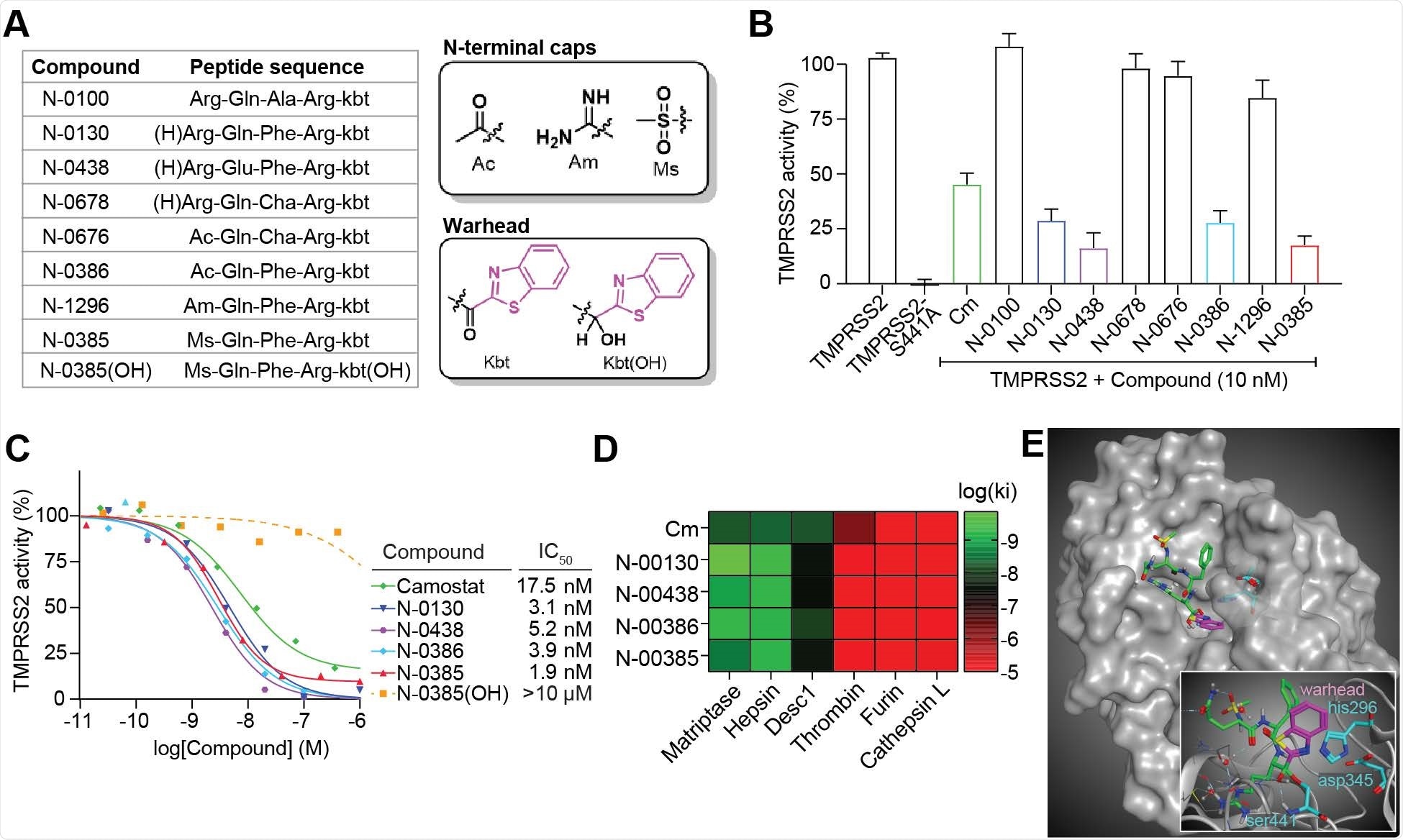
Ketobenzothiazole-based small-molecule peptidomimetics are potent TMPRSS2 inhibitors. (A) List of the peptidomimetic compounds used in this study along with their respective sequences. The structures of the N-terminal caps, the ketobenzothiazole warhead, and the alcohol ketobenzothiazole are shown on the right. (B) Vero E6 cells were transfected with either an empty vector (mock), TMPRSS2 wild type (WT), or the inactive mutant TMPRSS2-S441A for 24 hr. Indicated compounds (10 nM) were added concomitantly with a fluorogenic substrate on cells for an additional 24 hr before fluorescence reading. Relative TMPRSS2 activity was measured using the mock-subtracted fluorescence and reported as the percentage of residual activity relative to the saline-treated cells (0.01% DMSO). n = 3. (C) Dose-response curves were generated for the indicated compounds (n ≥ 3) using the assay described in (A) and IC50 values were determined using nonlinear regression analysis. Representative IC50 curves are shown. (D) Specificity of selected compounds toward other serine proteases is shown. Data are represented as log (Ki), n ≥ 3 and represented as a heat map. (E) Large: Docking of N-0385 (green, warhead in purple) in the binding pocket of TMPRSS2 (homology model). Residues of the catalytic triad are shown in cyan. Small: Interaction of N-0385 with TMPRSS2 residues. N-0385 forms a covalent bond with catalytic triad residue Ser441.
Study significance
The study identifies a novel small molecule TMPRSS2 inhibitor (N-0385) with high potency antiviral efficacy against SARS-CoV-2. The compound can inhibit viral replication by 99%, even at nanomolar concentrations. When intranasally administered to mice at the early infection phase, the compound shows high efficacy in preventing SARS-CoV-2-induced pathologies and mortality.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Shapira T. 2021. A novel highly potent inhibitor of TMPRSS2-like proteases blocks SARS-CoV-2 variants of concern and is broadly protective against infection and mortality in mice. BioRxiv. https://www.biorxiv.org/content/10.1101/2021.05.03.442520v1
- Peer reviewed and published scientific report.
Shapira, Tirosh, I. Abrrey Monreal, Sébastien P. Dion, David W. Buchholz, Brian Imbiakha, Andrea D. Olmstead, Mason Jager, et al. 2022. “A TMPRSS2 Inhibitor Acts as a Pan-SARS-CoV-2 Prophylactic and Therapeutic.” Nature, March, 1–13. https://doi.org/10.1038/s41586-022-04661-w. https://www.nature.com/articles/s41586-022-04661-w.