Researchers in the UK have developed a protein-based subunit vaccine directed against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that could serve as an alternative to the mRNA-based vaccines currently approved for protecting against coronavirus disease 2019 (COVID-19).
The team – from the University of Liverpool and the MRC Laboratory of Molecular Biology in Cambridge – says the sub-unit approach offers distinct advantages over mRNA-based vaccines in terms of the ease and cost of production, the robustness of material and the potency of protection.
The researchers showed that a ferritin-like protein from the archaeon Sulfolobus islandicus coupled with different antigens from SARS-CoV-2 formed extremely stable vaccine nanoparticles that completely protected mice from SARS-CoV-2-associated pneumonia and disease after just a single immunization.
“Our data highlight that multimerized SARS-CoV-2 subunit vaccines are a highly efficacious modality, particularly when combined with an ultra-stable scaffold,” says Jan Löwe and colleagues.
The team says further research is needed to develop the scaffold into a bona fide vaccine for protection against SARS-CoV-2 and other viruses.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
More about the currently approved vaccines
Since the COVID-19 outbreak first began in late December 2019, intense efforts to develop vaccines against SARS-CoV-2 quickly led to the emergency use authorization of several candidates.
These vaccines encode the viral spike protein, which mediates the initial stage of the infection process by attaching to the host cell receptor angiotensin-converting enzyme 2 (ACE2) via its receptor-binding domain (RBD).
The spike protein and its RBD are primary targets of the neutralizing antibodies generated following vaccination or natural infection.
So far, the vaccines approved for emergency use have been shown to be highly effective at protecting against symptomatic COVID-19 and the mass vaccination programs being rolled out in many countries have reduced the number of COVID-19 hospitalizations and deaths.
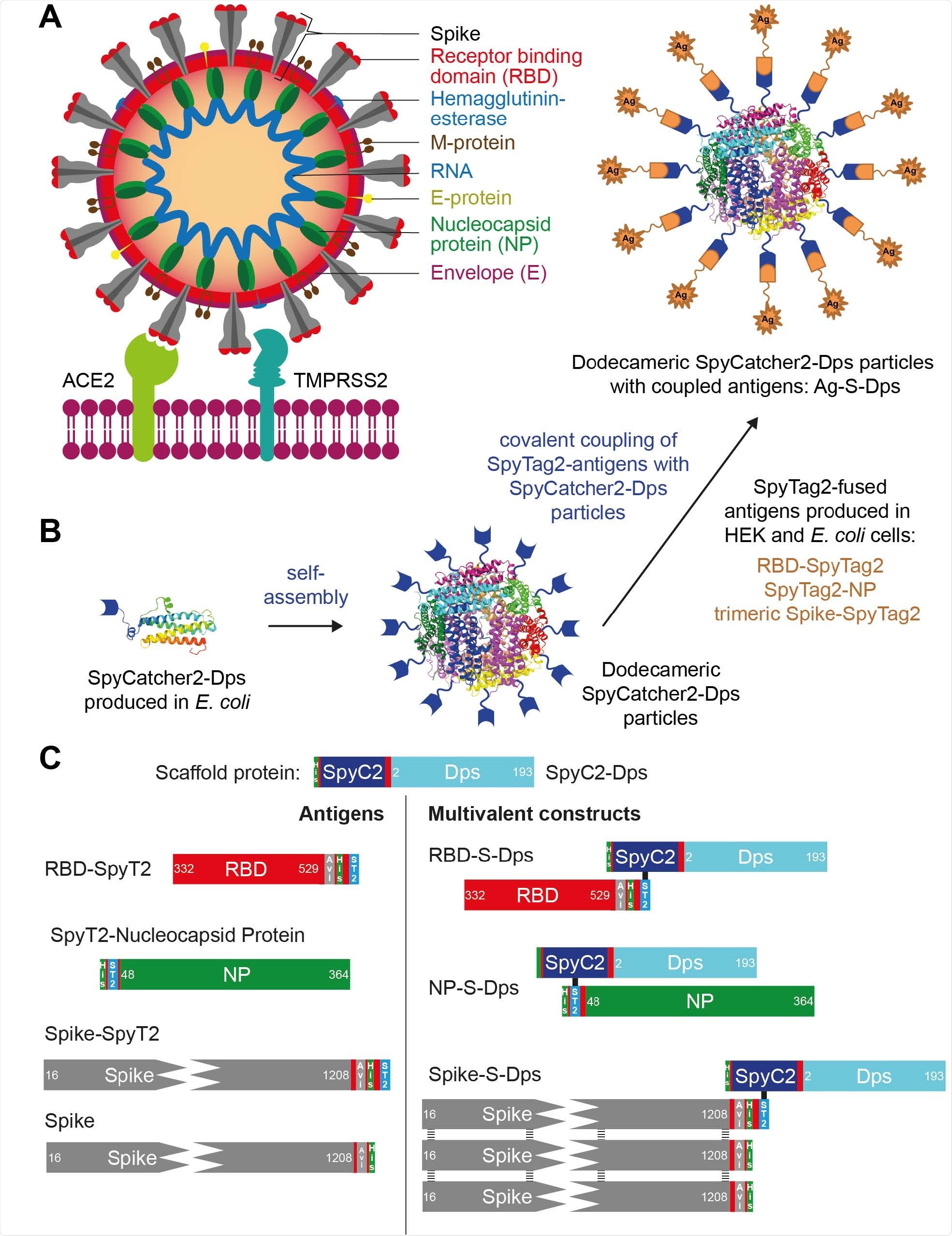
Overview of the multimerisation strategy employed and the antigens and scaffold used. A) Cartoon representation of SARS-CoV-2 binding to a human cell membrane. B) Schematic diagram of the Sulfolobus islandicus Dps and SpyCatcher2-based display and multimerisation strategy employed in this study. C) Diagram of the proteins used in this work. SpyC2 is the SpyCatcher2 domain and SpyT2 is the peptidic SpyTag2 that becomes covalently linked to SpyC2 upon simple mixing. Stabilized, trimeric Spike/Spike-SpyT2 contained on average only one SpyT2 tag in order to avoid uncontrolled oligomerisation when coupled to Dps.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Where do protein-based subunit vaccines come in?
Most of the vaccines approved so far are mRNA-based, vector-based, or DNA vaccines. Vector- and RNA-based vaccines can be rapidly developed since they deliver the coding sequence for the spike antigen rather than the immunogen itself.
“Currently, only one vaccine candidate in late-phase trials is a protein-based subunit vaccine,” says Löwe and colleagues.
The researchers say that some subunit vaccines are amenable to processes such as lyophilization, which eliminates the need for complex storage and cold-chain infrastructures.
“As such, they provide substantial advantages over nucleic-acid-based vaccines in the quest for complete and global vaccination,” they write.
Subunit vaccines also offer advantages over nucleic-acid-based vaccines in terms of cost, simplicity, production capacity, transport and administration.
Another challenge faced in global vaccination efforts is the emergence of SARS-CoV-2 variants, some of which are more resistant to vaccine-elicited immune responses.
“It is anticipated that in the future, several different types of vaccines will be required to cope with emerging variants of SARS-CoV-2,” says the team.
What did the researchers do?
Aiming to develop a stable and efficient scaffold that would enable the display and multimerization of various SARS-CoV-2 antigens, the team covalently linked SARS-CoV-2 proteins expressed in mammalian and bacterial cells with bacterially-expressed ferritin-like Dps protein from the archaeon Sulfolobus islandicus.
“Multimerization has been used for many years to increase the immunogenicity of different antigens through multivalency, and this approach has also been recently shown to work well with SARS-CoV-2 antigens,” say the researchers.
Löwe and colleagues found that linkage of the SARS-CoV-2 RBD to the Dps (RBD-S-Dps) formed extremely stable, multivalent vaccine nanoparticles that remained intact, even following lyophilization.
The team found that immunization with RBD-S-Dps was highly effective at eliciting neutralizing antibodies that inhibited cell entry in vitro.
In addition, immunization experiments in mice found that RBD-S-Dps elicited a higher antibody titer and an enhanced neutralizing antibody response, compared with the RBD alone.
Furthermore, a single immunization with the multivalent vaccine completely protected mice from serious illness after they were challenged with SARS-CoV-2 and efficiently cleared the virus from the lungs.
“A viable choice as a vaccine modality for SARS-CoV-2”
“Here we have shown that the ferritin-like protein Dps from the hyperthermophile S. islandicus possesses exceptional qualities as a SARS-CoV-2 subunit vaccine scaffold,” says Löwe and colleagues.
The researchers say that further research is needed to develop the Dps-scaffold into a bona fide vaccine for SARS-CoV-2 and other viruses. Replicating the robust neutralizing antibody response and high level of protection that was observed here in humans will be crucial, they add.
“Future work notwithstanding, our data add to a body of evidence that subunit-based vaccines represent a viable choice as a vaccine modality for SARS-CoV-2,” writes the team.
“Whilst other vaccine formats are significantly more advanced, subunit approaches such as Dps offer distinct advantages in simplicity of production, requiring no proprietary technology, robustness of material and potency of protection.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Löwe J, et al. Single-dose immunisation with a multimerised SARS-CoV-2 receptor binding domain (RBD) induces an enhanced and protective response in mice. bioRxiv, 20201. doi: https://doi.org/10.1101/2021.05.18.444622, https://www.biorxiv.org/content/10.1101/2021.05.18.444622v1
- Peer reviewed and published scientific report.
Salzer, Ralf, Jordan J. Clark, Marina Vaysburd, Veronica T. Chang, Anna Albecka, Leo Kiss, Parul Sharma, et al. 2021. “Single‐Dose Immunisation with a Multimerised SARS‐CoV‐2 Receptor Binding Domain (RBD) Induces an Enhanced and Protective Response in Mice.” FEBS Letters 595 (18): 2323–40. https://doi.org/10.1002/1873-3468.14171. https://febs.onlinelibrary.wiley.com/doi/10.1002/1873-3468.14171.