As 2021 approaches its midway point, the coronavirus disease 2019 (COVID-19) pandemic shows no sign of subsiding in many parts of the world. With successive variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerging, some showing evidence of increased transmissibility and severity of disease, the need for effective and safe antivirals remains as urgent as ever.
A new paper by an international team of researchers describes experimental evidence of the antiviral efficacy of a marine compound called plitidepsin against SARS-CoV-2. The findings show that plitidepsin inhibits SARS-CoV at low concentrations, as observed by a thousand-fold reduction in genomic RNA and viral titers in cells exposed to the virus in culture when treated.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
A preprint version of the team's study is available on the medRxiv* server, while the article undergoes peer review.
Background
The marine habitat is friendly to many viruses, especially those like SARS-CoV-2, with single-stranded ribonucleic acid (RNA) genomes. This prompted the search for potential antiviral natural compounds, resulting in the identification of plitidepsin.
This is a cyclic compound that comes from a colony-forming sea squirt called Aplidium albicans, from the Mediterranean Sea. This peptide inhibits the activity of the host protein called eukaryotic translation elongation factor 1A (eEF1A), a very common eukaryotic protein synthetic factor. It is hijacked by many viruses to facilitate their replication within the host cell.
SARS-CoV-2 nucleocapsid (N) protein is important in viral particle assembly. It also interacts with eEF1A, and when the latter is blocked from expression, virus replication is impaired.
Plitidepsin was developed as a cancer drug, and has been approved for the treatment of relapsed or refractory multiple myeloma, in combination with dexamethasone, by the Australian Therapeutic Goods Administration (TGA).
Plitidepsin inhibits SARS-CoV-2 in vitro
The current study was a proof-of-concept trial of plitidepsin in patients hospitalized in COVID-19.
It inhibited 50% of cytopathic effects (CPE) produced by SARS-CoV-2 (50% inhibitory concentration, IC50) without harming the cells themselves, at 0.038μM.
This nanomolar efficacy was also seen with the newer viral variants, including B.1.1.7, in both human lung cells and gastrointestinal cell lines. The researchers also observed that "plitidepsin was more effective against both variants than remdesivir."
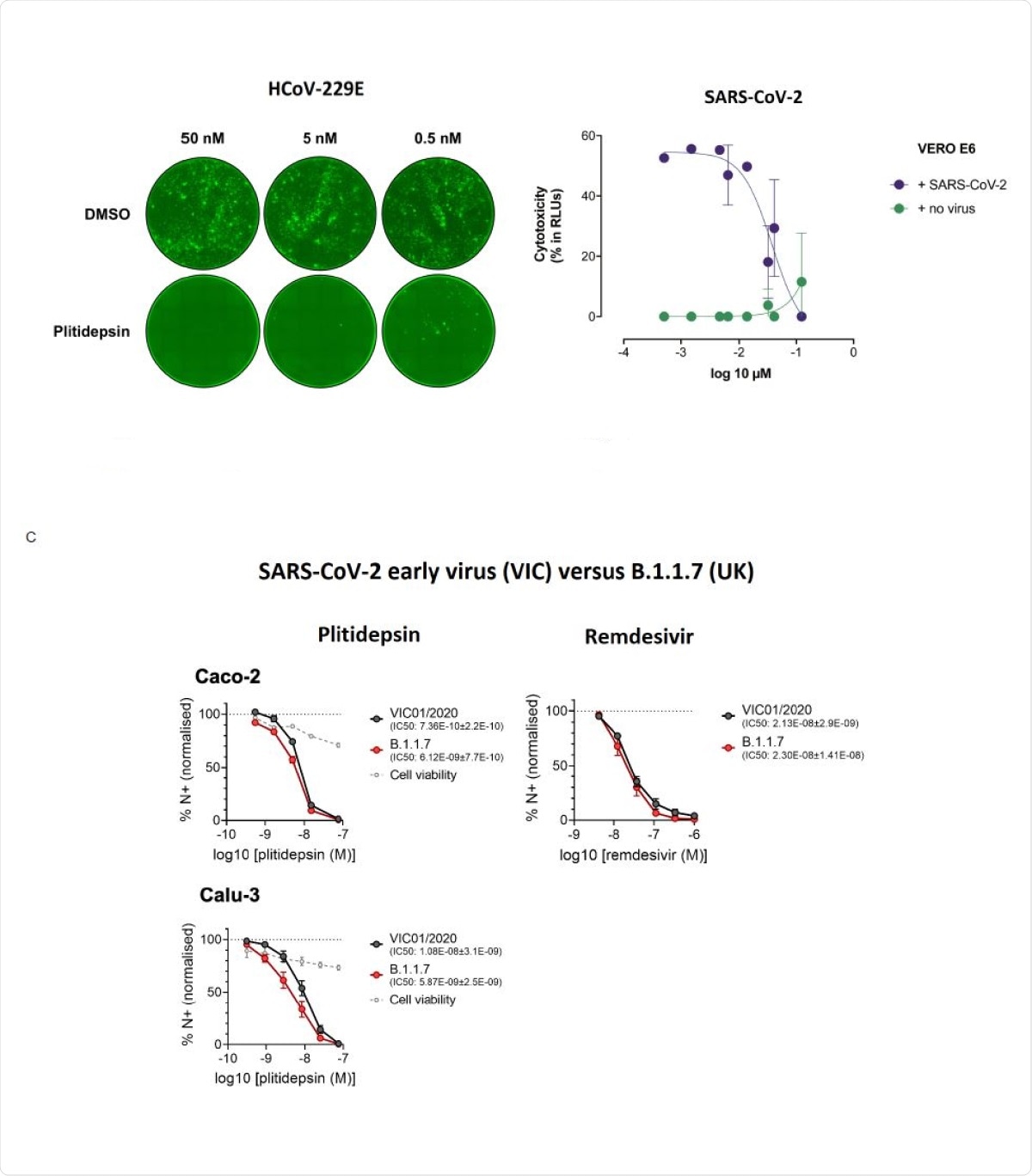
Plitidepsin shows strong antiviral activity in vitro against different coronavirus species and variants. (A) HCov-229E-GFP-infected Huh-7 cells were treated with indicated doses of plitidepsin or DMSO. All cells were treated 8 hours after infection and fluorescent foci were analyzed at 48 hours. (B) Cytopathic effect on Vero E6 cells exposed to a fixed concentration of SARS-CoV-2 in the presence of increasing concentrations of plitidepsin. Drug was used at a concentration ranging from 5 nM to 100 µM. Non-linear fit to a variable response curve from one representative experiment with two replicates is shown (blue), excluding data from drug concentrations with associated toxicity; cytotoxicity in the absence of virus is also shown (green). (C) Calu-3 and Caco-2 cells were pre-treated with remdesivir or plitidepsin at the indicated concentrations or DMSO control at an equivalent dilution for 2 h before SARS-CoV-2 infection. Cells were harvested after 24h for analysis, and viral infection measured by intracellular detection of SARS-CoV-2 nucleoprotein by flow cytometry. Tetrazolium salt (MTT) assay was performed to verify cell viability.
Testing clinical efficacy
Extrapolating from the in vitro results, the researchers concluded that target plitidepsin concentrations in the human lung and in plasma could be reached at 1.5 mg to 2.5 mg. With these concentrations, the plasma concentrations remained above the IC90 for half and most of the treatment period, respectively.
Another researcher showed that for an IC90 of 0.88 nM, the plasma concentration of the drug must be kept >0.18 μg/L.
Study details
Following these results, the proof-of-concept APLICOV-PC study examined the effects of three dosage levels, namely, 1.5 mg/day, 2.0 mg/day, and 2.5 mg/day as single doses, over three days. The drug was administered intravenously over 90 minutes to hospitalized COVID-19 patients.
Dexamethasone was used both for premedication prior to plitidepsin infusion, as well as an independent therapy for COVID-19, in about two-thirds of the patients in this study. After treatment with plitidepsin for three days, other agents were used in 16% of patients, such as remdesivir and tocilizumab.
The multi-center Spanish trial included 45 patients, of whom 44 completed it. On average, patients were 52 years old, and two-thirds were males. Over 80% had other illnesses, mostly obesity, hypertension and diabetes.
About half the patients had moderate COVID-19, with over a third showing severe disease. Bilateral pneumonia was present in >70%. All dosage groups had similar proportions of bilateral pneumonia and similar viral loads.
Plitidepsin found to be safe
One patient had a severe hypersensitivity reaction to the first dose and stopped treatment. For this reason, the remaining patients were given dexamethasone and antihistamines before plitidepsin infusions began.
Over half the patients had adverse effects, and one in three had grade 3 adverse events, with the highest prevalence in the 2.5 mg/day dosage cohort and the lowest in the 2 mg/day cohort.
Only two adverse events were traced to the drug, namely, the hypersensitivity reaction, in a patient on 1.5 mg/day, and diarrhea, in the 2.5 mg/day cohort. The others were attributed to the viral illness itself. No grade 4 events occurred.
Three patients died, all of whom had severe COVID-19 at admission to the study. None of the deaths were due to the drug.
80% of patients discharged by day 15
The drug's efficacy was assessed on day 8 and day 15 from the start of the 3-day treatment regimen. Over 55% of patients who received plitidepsin were discharged by day 8, and over 80% by day 15.
At the second time point, the viral load was reduced by 4.2 logs and became undetectable at an average of 13 days. As expected, severe COVID-19 patients took longer to reach this stage (15 days).
At day 8, 43% of patients in the 1.5 mg/day were discharged, vs 67% at 2.5 mg/day. Even more significantly, all patients with moderate COVID-19 on the highest dosage were discharged by day 8, relative to 43% and 38% of those on 2 mg/day and 1.5 mg/day, respectively.
Patients with mild and moderate COVID-19 were discharged at a median of 7 days, but 14 days in those with severe COVID-19 at the time of entry into the study.
Other effects
The study also suggests that at higher dosages, inflammation resolved more rapidly, due either to reduced viral replication or as the effect of the drug itself. Moreover, the researchers postulate that the emergence of plitidepsin resistance is unlikely since it targets a host protein and not a viral component.
This may make the drug equally effective against all variants of the virus. This hypothesis, as well as the findings of the current study, must be confirmed in larger placebo-controlled trials. One such phase 3 trial is ongoing in Europe.
What are the implications?
This early proof-of-concept trial supports earlier in vitro and in vivo tests that show potent inhibition of SARS-CoV-2 infection by plitidepsin. This data promotes the need for advanced clinical trials to validate the finding of plitidepsin efficacy in enhancing recovery among patients hospitalized with moderate COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Varona, J. F. et al. (2021). Plitidepsin has a positive therapeutic index in adult patients with COVID-19 requiring hospitalization. medRxiv preprint. doi: https://doi.org/10.1101/2021.05.25.21257505, https://www.medrxiv.org/content/10.1101/2021.05.25.21257505v1
- Peer reviewed and published scientific report.
Varona, Jose F, Pedro Landete, Jose A Lopez-Martin, Vicente Estrada, Roger Paredes, Pablo Guisado-Vasco, Lucia Fernandez de Orueta, et al. 2022. “Preclinical and Randomized Phase I Studies of Plitidepsin in Adults Hospitalized with COVID-19.” Life Science Alliance 5 (4): e202101200. https://doi.org/10.26508/lsa.202101200. https://www.life-science-alliance.org/content/5/4/e202101200.