A newly discovered molecule central to the assembly of HIV, termed IP6, may provide valuable insight into the development and function of viruses and how to combat them.
In a study published in the journal of Nature Communications, researchers from Cornell University and the University of Missouri discovered a key molecule important in the assembly of retroviruses.
In 2019 alone, 690 000 people died because of human immunodeficiency virus (HIV) infection, a debilitating and lethal disease that can lead to AIDS (acquired immunodeficiency syndrome). HIV remains a particularly efficient disease with no effective cure, yet scientists are gradually able to study its close relatives to better understand its infectivity, development, and structure.
"If you want to know the enemy, you have to know all its friends," states first author Martin Obr, a postdoc at the Schur group at IST Austria. Together with his colleagues, he considered a virus of the same family as HIV causing cancer in poultry, the Rous sarcoma virus.
The researchers studied the blueprint of this species as a model for viral infection, investigating how viruses have perfected the ability to insert genetic material and multiply within healthy human cells. Such abilities involve a host of skills from avoiding immune system detection to high reproductive rates, all with a refined precision, resulting in deadly viruses.
Breaking down how viruses can accomplish such tasks involves an examination of their components that once assembled can form a functioning molecular machine.
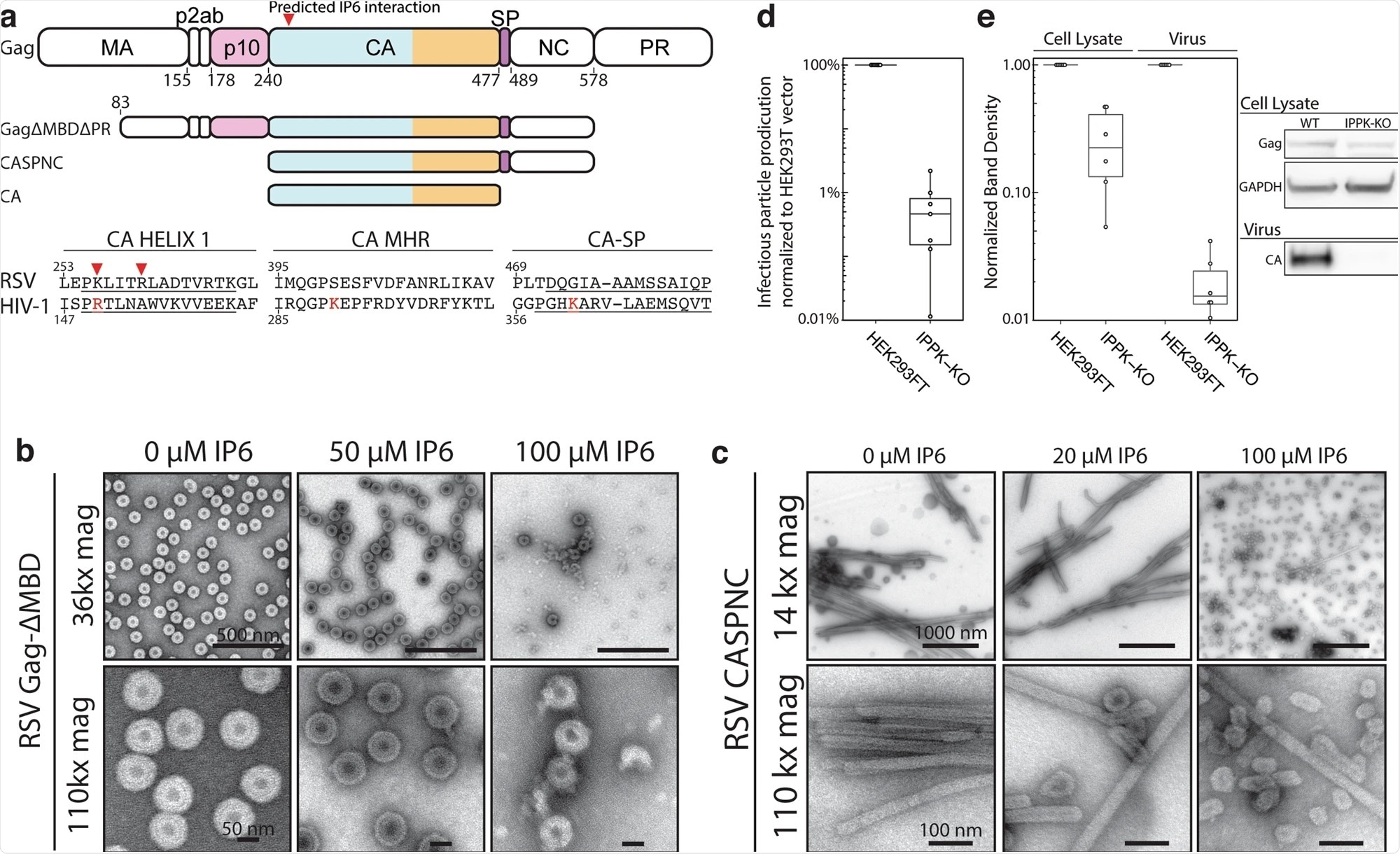
Effect of IP6 on RSV in vitro and in cells. Image Credit: https://www.nature.com/articles/s41467-021-23506-0/figures/1
A new actor in virus stabilization and protection
Researchers focused on the late phase of retrovirus replication of the Rous sarcoma virus, a key point of viral development as the virus transitions towards infectious stages. Viral particles first arise in an immature state, unable to infect, and then form a capsid, or protected shell, around its DNA, before becoming infectious.
This capsid consists of a protein organized in hexamers and a few pentamers and can protect the genetic information of the virus. In this study, researchers discovered that a specific molecule plays a major role in stabilizing the shell within the Rous sarcoma virus, termed IP6.
When building a car, you have all these big metal parts, like the hood, the roof, and the doors - the screws are connecting everything. In our case, the big parts are the capsid proteins and the IP6 molecules are the screws,"
Obr
IP6 acts as a key stabilizer for this virus, ensuring adequate protection during its development into an infectious state. This is a pivotal role as viral development requires stability to replicate and mature.
“If the protective shell is not stable, the genetic information of the virus could be released prematurely and will be destroyed, but if it's too stable the genome can't exit at all and, therefore, becomes useless," says Assistant Professor Florian Schur.
Findings from this study have demonstrated that IP6 is crucial not only for the assembly of HIV but also for other viruses of the same family. Therefore, the small molecule has proven to be a fundamental component of the virus life cycle.
The shapes, sizes, and potential adaptability of capsids
The team then used cryo-electron tomography to examine samples of the virus under its natural state and found that capsid proteins are highly variable in morphology. This abnormality poses many questions including the reason underlying such variation.
Now we ask ourselves: Why does the virus change the shape of its capsid? What is it adapting to?"
Previous studies have shown that it could be due to the differences in the infectivity of the virus particles but "Whatever happens, happens for a reason but there is no clear answer yet," says Florian Schur.
This provides a potential trajectory for future research, which could be particularly useful in understanding the life cycle, replication, and overall life history of viruses. Aspects of these highly efficient pathogens remain an enigma, and scientists may be able to elucidate some of the answers using emerging technology and molecular methods.
Additionally, such findings will further support the identification, protection, and treatment of viral infections, as even if IP6 is specialized to key viral families, similar agents may be present across other viruses. Interrupting such developmental stages may be a focal target for treatments, as "It is a long way from an infected cell to the mature virus particle that can infect another cell," explains Martin Obr, suggesting a potential opportunity for destabilizing key agents of viral development.
Source:
- Martin Obr, Florian K. M. Schur et al. 2021. Structure of the mature Rous sarcoma virus lattice reveals a role for IP6 in the formation of the capsid hexamer. Nature Commmunications. DOI: 10.1038/s41467-021-23506-0