The optimum dosing interval between COVID-19 vaccines is set by the manufacturer and is advised by government health agencies in their respective country of concern, as is the decision to distribute a second dose or rely on the lesser immunity granted by the first dose only.
The Canadian National Advisory Committee on Immunization has recommended setting the maximum period between doses at 16 weeks, and researchers from several Canadian universities have recently investigated the validity of this recommendation and the progress of the vaccination scheme in general by analyzing data from over 300,000 patients at each stage of vaccination: non-vaccinated, first or both doses.
The report has recently been published on the preprint server medRxiv* by Kwong et al. (May 28th, 2021) and focused on the Pfizer-BioNTech or Moderna vaccines, confirming the supposed greater efficacy in preventing infection or severe outcomes following the administration of two doses.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
How was the study performed?
All adult Ontarians eligible for provincial insurance tested for SARS-CoV-2 between December 14th, 2020 and April 19th, 2021 were included in the study, with those who had received a positive test before this time excluded.
Only individuals with at least one presenting COVID-19 symptom were included, and each positive SARS-CoV-2 case was confirmed by PCR testing. Information regarding the specific variant of SARS-CoV-2 an individual was infected with was also recorded by genome sequencing and identification of N501Y and E484K mutations from February and March onwards, respectively.
The E484K mutation is indicative of the variants of concern B.1.1.7, while the presence of both mutations indicates lineage B.1.351 or P.1, which were pooled together due to low infection counts in this study.
Individual socioeconomic factors were also taken into consideration, including the number of residents per home, the nature of occupancy, the risk of contracting the disease, and income, and the presence of comorbidities was weighed against the risk of death from COVID-19 regardless of vaccination.
Of all of the participants in the study that had tested positive for SARS-CoV-2 during the period concerned, over 60% were asymptomatic, with test-positive cases being more likely to be male, have had no other COVID-19 tests within three months, live in lower-income areas with more co-residents, and are more likely to be an essential worker, while the opposite of each is true for those that have received at least one dose of vaccine.
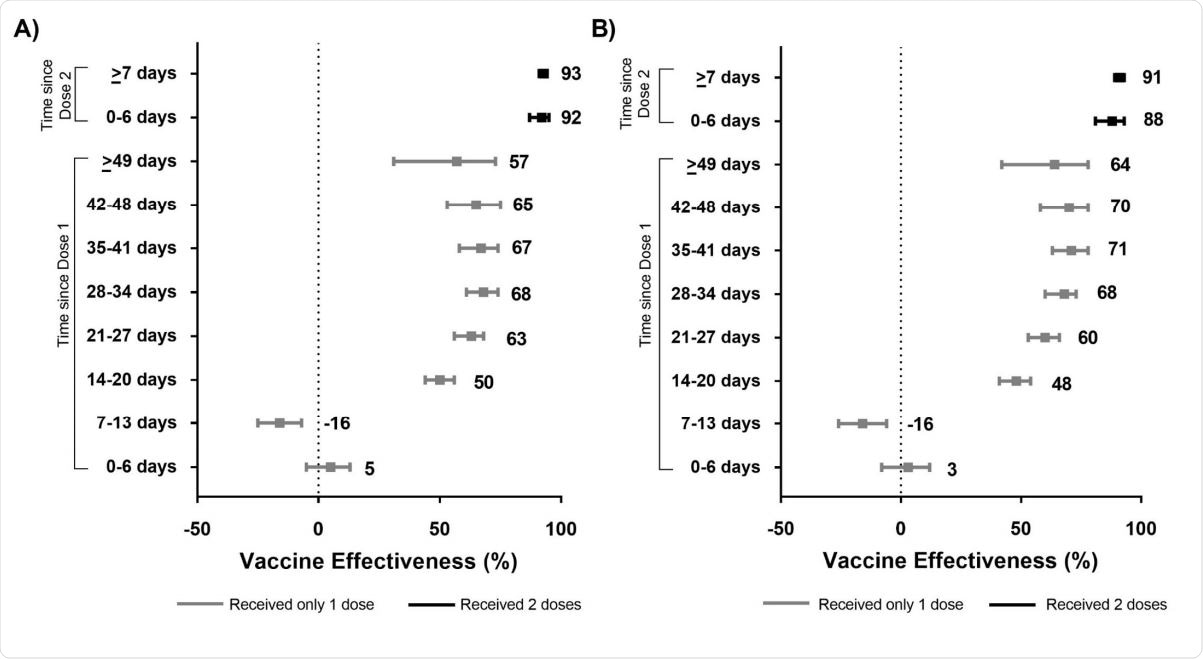
Unadjusted (panel A) and adjusted (panel B) vaccine effectiveness estimates of COVID-19 mRNA vaccines (BNT162b2, mRNA-1273) against laboratory-confirmed symptomatic SARS-CoV-2 infection by various intervals, between 14 December 2020 and 19 April 2021 in Ontario, Canada.
How effective is vaccination?
Vaccine effectiveness against symptomatic infection was determined to be around 60%, rising from 48% at two weeks to 71% at 4/5 weeks. The group observed a 16% increase in the risk of symptomatic infection one to two weeks after the first dose, which the group suggests could result from increased SARS-CoV-2 exposure shortly before, during, or after vaccination.
Individuals could be exposed upon traveling to and receiving the dose or by an increased occurrence of risky behavior encouraged by the reassurance of protection. Cases of undetected infection could also apply, where individuals just recently infected with SARS-CoV-2 receive the vaccine but do not yet produce a positive PCR test.
The vaccines investigated began being distributed in Canada in December 2020, prioritizing high-risk groups such as the elderly and front line workers.
A three or four-week interval between first and second doses was initially followed. However, this was later relaxed for some groups to almost double this period in late January due to vaccine supply issues, and has since been relaxed further to 16 weeks since April.
Vaccine effectiveness rose from just 71% several weeks after the first dose to 91%, one week after receiving the second dose. The Moderna mRNA vaccine proved more effective than the Pfizer-BioNTech vaccine against symptomatic infection in the early weeks following the first dose, though rates were comparable after having both doses. Vaccine effectiveness was also higher amongst younger adults and those with fewer comorbidities, while the earlier variant of concern B.1.1.7 was better suppressed than the B.1.351 pr P.1 variants. Importantly, though, the vaccines proved effective against both variants of concern. Vaccine effectiveness was achieved more quickly and completely for younger adults, though once both vaccines were administered, rates of vaccine effectiveness were comparable across all groups.
Similarly, lessened rates of severe outcomes of hospitalization or death were also recorded, lowering by 70% two weeks after the first dose and 91% one month later, rising to 98% one week after the second dose.
This study demonstrates the positive effect that widespread vaccination programs are having against the COVID-19 pandemic, demonstrably lowering infection, hospitalization, and death rates.
The authors emphasize that individuals that have received the first dose only should continue to adhere to non-pharmaceutical interventions and social distancing procedures, as immunity takes several weeks, and preferably a second dose, to develop reliably.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada, Hannah Chung, Siyi He, Sharifa Nasreen, Maria E. Sundaram, Sarah A. Buchan, Sarah E. Wilson, Branson Chen, Andrew Calzavara, Deshayne B. Fell, Peter C. Austin, Kumanan Wilson, Kevin L. Schwartz, Kevin A. Brown, Jonathan B. Gubbay, Nicole E. Basta, Salaheddin M. Mahmud, Christiaan H. Righolt, Lawrence W. Svenson, Shannon E. MacDonald, Naveed Z. Janjua, Mina Tadrous, Jeffrey C. Kwong, medRxiv, 2021.05.24.21257744; doi: https://doi.org/10.1101/2021.05.24.21257744, https://www.medrxiv.org/content/10.1101/2021.05.24.21257744v1
- Peer reviewed and published scientific report.
Chung, Hannah, Siyi He, Sharifa Nasreen, Maria E. Sundaram, Sarah A. Buchan, Sarah E. Wilson, Branson Chen, et al. 2021. “Effectiveness of BNT162b2 and MRNA-1273 Covid-19 Vaccines against Symptomatic SARS-CoV-2 Infection and Severe Covid-19 Outcomes in Ontario, Canada: Test Negative Design Study.” BMJ (Clinical Research Ed.) 374 (August): n1943. https://doi.org/10.1136/bmj.n1943. https://www.bmj.com/content/374/bmj.n1943.