The devastating medical and economic impacts of the coronavirus disease 2019 (COVID-19) pandemic have triggered an unprecedented global effort toward the development of effective vaccines to prevent infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus responsible for COVID-19.
To date, multiple vaccines have been approved in several nations around the world. Each of these vaccines is parenterally administered, which can pose various logistical challenges. Whether these vaccines offer adequate protection at the mucosal sites of entry of the virus also remains questionable.
Currently approved vaccines, as well as other vaccine candidates that are still in development, target the SARS-CoV-2 spike (S) protein, which is a surface protein that plays a key role in the entry of SARS-CoV-2 into the host cell.
 Study: Oral subunit SARS-CoV-2 vaccine induces systemic neutralizing IgG, IgA and cellular immune responses and can boost neutralizing antibody responses primed by an injected vaccine. Image Credit: Love Lego / Shutterstock.com
Study: Oral subunit SARS-CoV-2 vaccine induces systemic neutralizing IgG, IgA and cellular immune responses and can boost neutralizing antibody responses primed by an injected vaccine. Image Credit: Love Lego / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Developing an oral, multi-epitope SARS-CoV-2 vaccine
In a recent study published on the pre-print bioRxiv* server, a group of Israeli researchers discusses their progress towards developing an oral multi-antigen SARS-CoV-2 vaccine. This oral vaccine, which is otherwise known as MigVax-101, is comprised of the receptor-binding domain (RBD) of the SARS-CoV-2 S protein, 2 domains of the SARS-CoV-2 nucleocapsid protein (N), as well as the heat-labile enterotoxin B (LTB), which is a potent mucosal adjuvant.
In their work, the researchers assessed the mucosal, humoral, and cell-mediated immune responses of a 3-dose vaccination schedule, as well as a heterologous subcutaneous prime and oral booster regimen in mice and rats, respectively.
The mice that received the oral vaccine showed significantly enhanced production of virus-neutralizing antibody anti-S immunoglobulin G (IgG) and IgA. These mice also exhibited the N-protein-stimulated production of both interferon γ (IFN-γ) and interleukin-2 (IL-2) by T cells following dose 3 as compared to the control mice.
When administered as a booster dose to rats following parenteral priming with the SARS-CoV-2 S1 protein, the MigVax-101 vaccine induced significantly higher neutralizing antibody titers as compared to the rats that received the oral placebo booster. A single oral booster after 2 subcutaneous priming vaccine doses was found to induce serum IgG and mucosal IgA levels that were similar to the levels that were raised by 3 subcutaneous doses.
This study demonstrated the safety and immunogenicity of a 3-dose vaccination regimen using an oral multi-epitope SARS-CoV-2 vaccine in mice. Based on the observations from this study, the authors concluded that the oral LTB-adjuvanted, multi-antigen SARS-CoV-2 vaccine-elicited versatile cellular, humoral, and mucosal immune responses.
In addition to offering protection against SARS-CoV-2 infection through priming or booster programs, these vaccines also minimized the technical and logistical hurdles that are currently associated with the approved parenteral vaccines.
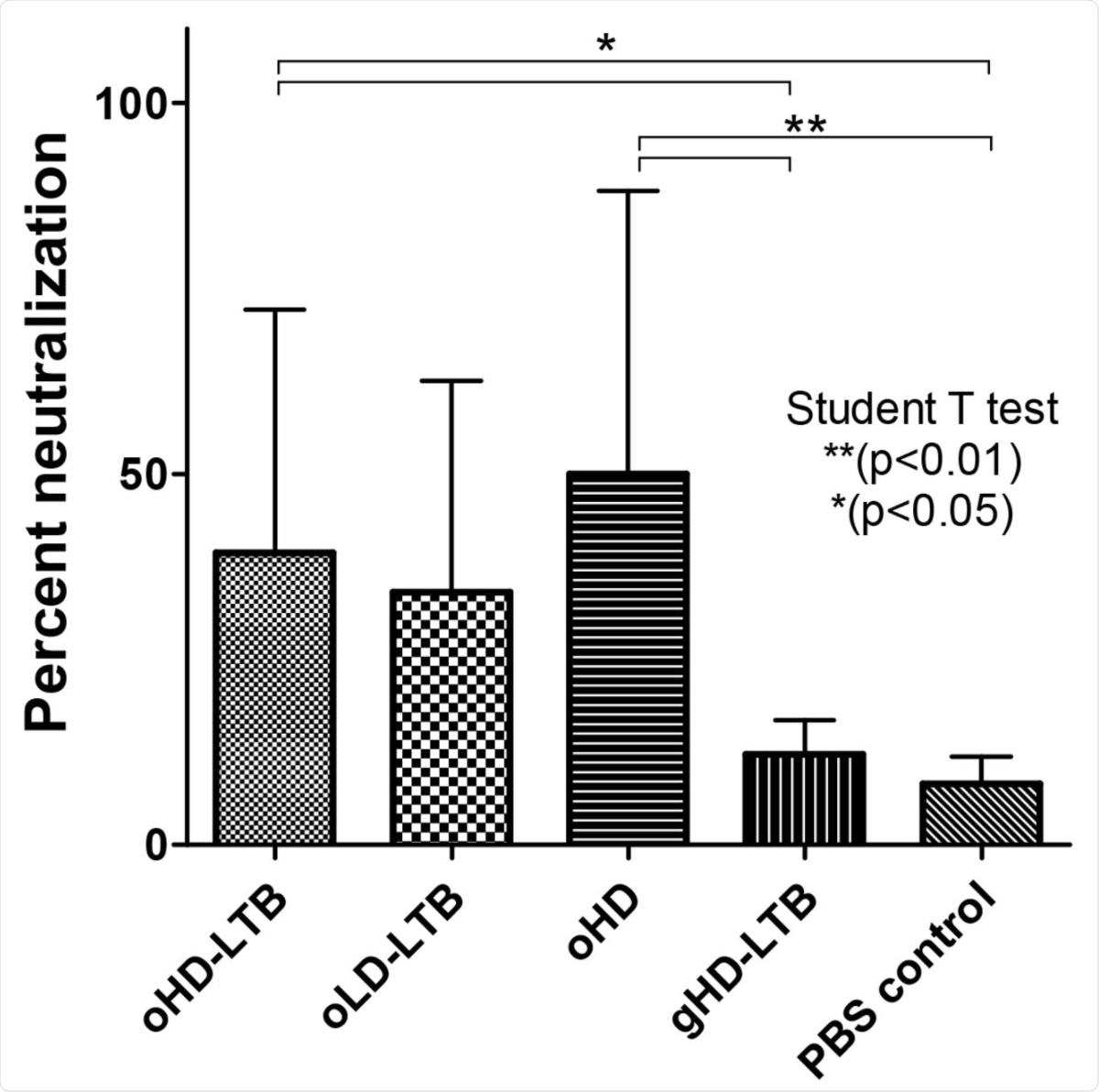
Neutralization potency following oral immunization: Mice (BALBc, 8-week-old, 5 males and 5 females per treatment group) were inoculated orally or by gavage on days 0, 14, and 28, with either high dose (oHD-LTB) or low dose (oLD-LTB) or high-dose vaccine without free LTB (oHD) per Figure 2. Mice received the high dose with free LTB (gHD-LTB) by gavage. Control mice were treated with an oral dose of PBS. Sera were diluted 10 fold and assessed for neutralizing activity using the cPass neutralization assay. The y-axis corresponds to the observed percentage of the binding inhibition of ACE2-RBD. The neutralization assay was performed in triplicate for each mouse serum; values show mean ± standard deviation. Student’s t-test was performed to determine p-values.
Advantages of an oral COVID-19 vaccine
The ongoing COVID-19 pandemic highlights the need for easily accessible vaccines like oral inoculations, which are user-friendly approaches that could easily be incorporated into a mass vaccination strategy. Given that SARS-CoV-2 is primarily transmitted via respiratory droplets, strong mucosal immunity might boost protection against nasal and/or oral entry of the virus, thus potentially advancing the development of herd immunity.
A vaccine that is administered through the oral route also overcomes many of the technical constraints that are often associated with the administration of traditional vaccines. Some of the ways in which oral vaccines avoid these challenges include eliminating the use of needles, which are extra devices that need to be distributed along with the vaccines. Additionally, this type of vaccine increases the patient’s comfort level, particularly for individuals who are afraid of needles. An oral vaccine also does not require trained individuals for their administration, which is particularly advantageous in developing countries.
The integration of the highly conserved N protein in this vaccine may lead to group-common immunity against many SARS-CoV-2 variants. Apart from the convenience of use, an oral vaccine booster may be beneficial for patients who experienced adverse reactions to the initial doses of injected vaccines.
“In addition to the potential clinical benefits of careful selection and combination of viral epitopes, the subunit vaccine carries several technical advantages over inactivated, attenuated or viral vector vaccines, including the possibility of mass-production in dedicated fermenters and no risk of contamination with residual pathogenic material.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Pitcovski, J., Gruzdev, N., Abzach, A., et al. (2021). Oral subunit SARS-CoV-2 vaccine induces systemic neutralizing IgG, IgA and cellular immune responses and can boost neutralizing antibody responses primed by an injected vaccine. bioRxiv. doi:10.1101/2021.06.09.447656. https://www.biorxiv.org/content/10.1101/2021.06.09.447656v1.
- Peer reviewed and published scientific report.
Pitcovski, Jacob, Nady Gruzdev, Anna Abzach, Chen Katz, Ran Ben-Adiva, Michal Brand-Shwartz, Itamar Yadid, et al. 2022. “Oral Subunit SARS-CoV-2 Vaccine Induces Systemic Neutralizing IgG, IgA and Cellular Immune Responses and Can Boost Neutralizing Antibody Responses Primed by an Injected Vaccine.” Vaccine, January. https://doi.org/10.1016/j.vaccine.2022.01.025.