The main organs affected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are the lungs, causing respiratory symptoms ranging from coughing and shortness of breath to severe lung injury.
However, there have been reports that the virus also affects other organs like the gastrointestinal (GI) tract, causing symptoms like vomiting and diarrhea. Viral RNA has also been found in feces, with samples testing positive even after nose or throat swabs have tested negative. However, it is not yet known how important the infection of the GI tract is in viral replication and transmission.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
During a viral infection, cells in the body generate an inflammatory and an interferon (IFN) response. To mount a response to a virus, almost all cells in the body produce and respond to type I IFN. Lung and intestinal epithelial cells also respond to type III IFN. Studies suggest that type III IFN response in intestinal cells is important in protecting against viral infection.
Treatment of lung and intestinal epithelial cells with type I and type III interferon has been reported to provide some protection against SARS-CoV-2 infection and interfere with virus replication. In intestinal cells, type III IFNs seem to play a more important role in controlling SARS-CoV-2 infection than type I interferons. However, little is known about the mechanism of this action.
Testing effect of interferons
In a new study, released as a preprint on the bioRxiv* server, researchers from Heidelberg University and the German Cancer Research Center report their results on how type I and III IFNs work as antivirals in the human gut.
The researchers infected colon carcinoma cells T84 with SARS-CoV-2. Some of the cells had either type I IFN receptors, type III IFN receptors, or both receptors knocked off. During early infection, the cells with type I receptors or both receptors knocked off had more infected cells compared to the cells with type III receptor knocked off or wild-type cells. However, about 12 hours after infection, cells with type III IFN receptors knocked off were more susceptible to infection. This suggests both types of receptors are important in SARS-CoV-2 infection.
They also found virus replication was higher in cells with both receptors knocked off, suggesting an absence of IFN signaling helps infection. Type I IFNs seem to play a role in mitigating infection early on, with type III IFNs playing a dominant role in later infection stages in human intestinal cells.
The concentration of IFNs also seems to play a role in inhibiting infection. A low concentration of type I IFN inhibition more SARS-CoV-2 than a higher concentration. Lower levels of type III IFNs also restricted SARS-CoV-2 infection to less than 5% of the cells. However, higher concentrations and a longer period of pretreatment were required to reduce genome copy number. Further tests revealed the inhibition mechanism is likely the prevention of virus translation by the IFNs.
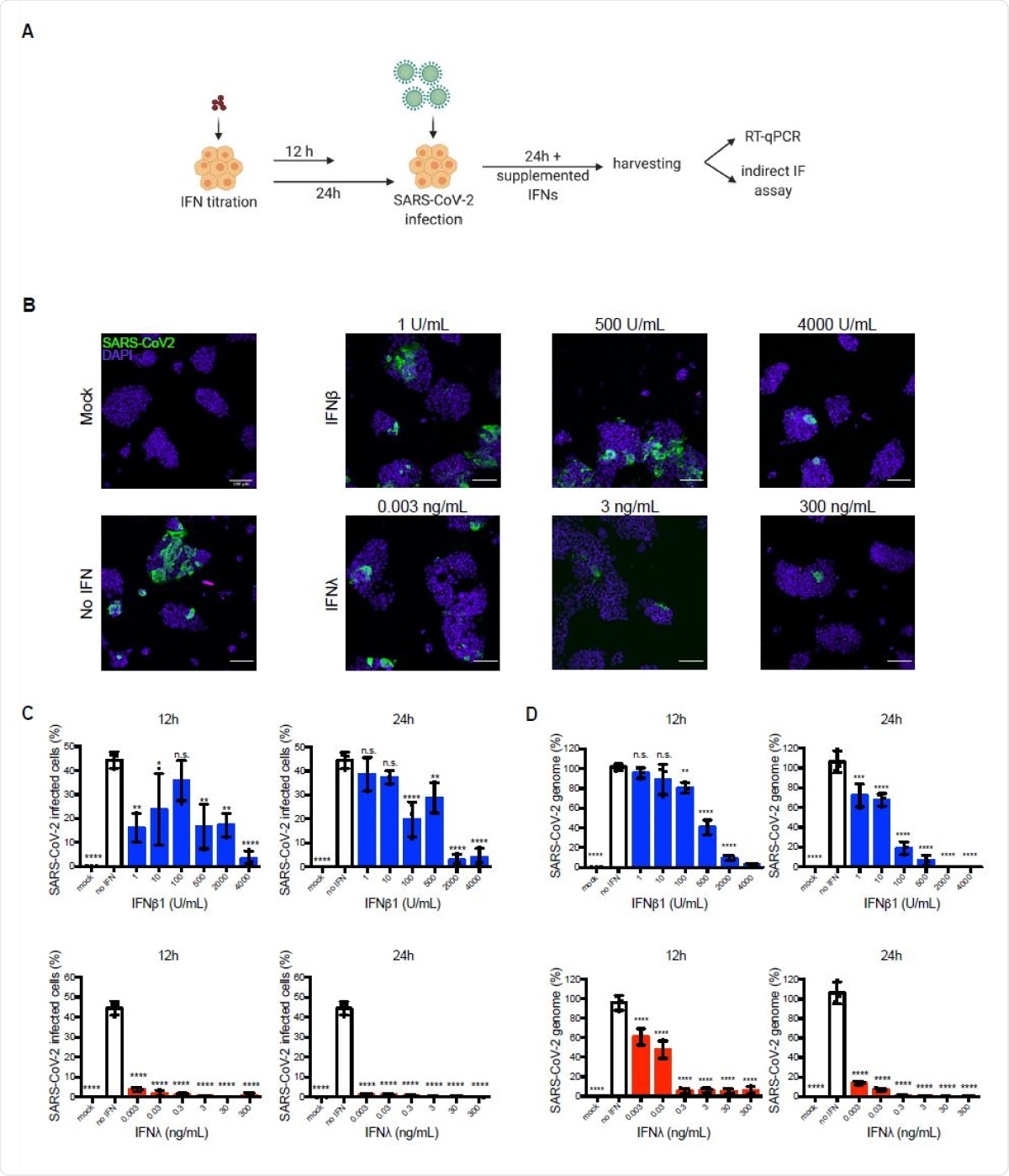
Exogenously added type I and III IFNs inhibit SARS-CoV-2 infection of hIECs in a concentration dependent manner. (A-D) WT T84 cells were mock-treated or pretreated with increasing concentration of type I (IFNβ1) and type III (IFNλ1/2/3) IFNs for 12 h and 24 h prior to infection. Cells were infected with SARS-CoV-2 using a MOI of 0.04. 24 hpi, cells were harvested to assay virus infection and replication. (A) Schematic of infection conditions. (B) Cells were fixed and indirect immunofluorescence was performed against the viral nucleocapsid (green). Nuclei were stained with DAPI (blue). Representative images are shown. Scale bars=100 μm. (C) The percentage of SARS-CoV-2-positive cells was quantified from images in (B). (D) RNA was harvested, and q-RT-PCR was used to evaluate the replication of the SARS-CoV-2 genome. Data are normalized to input and expressed as percentage, setting no-IFN treated cells to 100%. (C-D) Error bars indicate standard deviation. n = 3 biological replicates. n.s =not significant. P<0.05 *, P<0.01 **, P<0.001 ***, P <0.0001 **** as determined by ordinary one-way ANOVA with Dunnett’s multiple comparison test using nontreated infected cells as reference.
Type III interferon acts faster
Further experiments suggest that type I and type III IFNs reduce the spread of SARS-CoV-2 in human epithelial cells after virus entry by reducing the release of nucleocapsid and other viral proteins. This reduced the number of virus particles released to neighboring cells. However, further studies are needed to understand this mechanism better.
The kinetics of how the type I and type III IFNs work are also different, the former seeming to act early in the infection and the latter at later stages. The IFNs type also affects different viruses differently. The authors compared their effect on vesicular stomatitis virus (VSV) and SARS-CoV-2 and found low concentrations of type I IFN controls VSV infection, while low concentrations of type III IFN controls SARS-CoV-2 better.
Adding the IFNs to the cells after SARS-CoV-2 infection also helped inhibit viral replication. Type III IFN could provide protection against SARS-CoV-2 by inhibiting viral replication for more than 72 hours after withdrawing the IFN.
Thus, type III IFNs act quickly, need low concentration and shorter pretreatment periods, and offer long-lasting antiviral protection against SARS-CoV-2 in intestinal cells.
These observations suggest type III IFN treatment as a strong therapeutic candidate against SARS-CoV-2 infection of the human intestine,” write the study authors.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources