Acute lung injury (ALI) can lead to acute respiratory distress syndrome, which can cause significant and lethal effects for those infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
SARS-CoV-2, which is the causative agent of the coronavirus 2019 (COVID-19), was first reported in Wuhan, China in December of 2019. Since then, SARS-CoV-2 has infected over 182 million people worldwide and caused the deaths of almost 4 million people as of June 30, 2021.
 Study: The SARS-CoV-2 Spike Protein Subunit 1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Image Credit: Stefano Garau / Shutterstock.com
Study: The SARS-CoV-2 Spike Protein Subunit 1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Image Credit: Stefano Garau / Shutterstock.com
The COVID-19 pandemic has inspired international collaborations to work together in developing effective and safe vaccines in a rapid manner. While significant progress has been made in the development and distribution of these vaccines in many countries around the world, several challenges have limited the ability of many populations to also benefit from these medical advancements. Some of these challenges include delays in vaccine rollouts or stock availability, inefficient herd immunity, as well as the emergence of infectious viral strains.
As a result of these challenges, ongoing global efforts, particularly in the form of randomized clinical trials, have focused their attention on evaluating effective therapeutics that can mitigate the effects of COVID-19. To this end, researchers from collaborating universities in Virginia have recently utilized transgenic mice to explore the effects of SARS-CoV-2 on associated lung injury.
SARS-CoV-2 pathogenesis in lungs
The entry of SARS-CoV-2 into the host cell is dependent upon the interaction between the spike (S) protein, which is located on the surface of this virus, and the angiotensin-converting enzyme-2 (ACE-2) present on the host cell. However, it remains unknown whether the S protein is solely capable of modifying the vascular permeability of the lungs or producing lung injury in vivo.
Research investigating the pathogenicity of the virus is also limited due to the lack of available wild-type animal models that do not express ACE-2. As a result, transgenic mice which express the human ACE-2 gene under the control of the cytokeratin 18 promoter (K18-hACE2) were created. The development of this model has allowed researchers to reproduce the COVID-19 infection that is similar to that which is seen in infected humans.
In order to study the mechanisms by which COVID-19 can cause lung injury, the researchers of the current study, which was originally published in the American Journal of Physiology-Lung Cellular and Molecular Physiology, intratracheally instilled the S1 protein of SARS-CoV-2 into K18-hACE2 transgenic mice that overexpressed human ACE-2.
The researchers then observed the effects of COVID-19 and the signs of associated lung injury after 72 hours. The groups in this experiment included control K18-hACE2 transgenic mice that were provided with saline or the S protein, as well as the experimental wild-type mice which received the S1 protein.
After the S protein of SARS-CoV-2 binds to the host cell via the ACE-2 receptor, protease cleavage occurs. Following this, the S1 subunit of the S protein binds to the ACE-2 receptor and promotes the fusion of the viral membrane to the host cell and the release of viral genetic material into the cytoplasm through endocytosis.
The binding to ACE-2 can disrupt the renin-angiotensin signaling process, which can subsequently cause ACE-2 shedding. Notably, ACE-2 shedding has been associated with ALI, as well as increased vascular permeability and cytokine production.
Study findings
The aim of the current study was to resolve the uncertainty surrounding whether the S or S1 protein is responsible for stimulating the induction of local or systemic inflammation in vivo. The hypothesis of the study was that the intratracheal instillation of SARS-CoV-2 S1SP in K18-hACE2 mice would lead to ALI.
In their work, the scientists confirmed that the S1 protein does, in fact, produce COVID-19-like responses like ALI, when instilled intratracheally, which includes the production of a ‘cytokine storm’.
More specifically, the researchers found that the K18-hACE2 transgenic mice with the S1 protein experienced a decline in body weight, as well as a significant increase in white blood cell levels and protein concentrations in bronchoalveolar lavage fluid (BALF). There was also an upregulation of inflammatory cytokines in both the BALF and serum of mice. Histological analyses of the mouse lungs also confirmed the presence of ALI by detecting the activation of both the STAT3 and NFκB pathways.
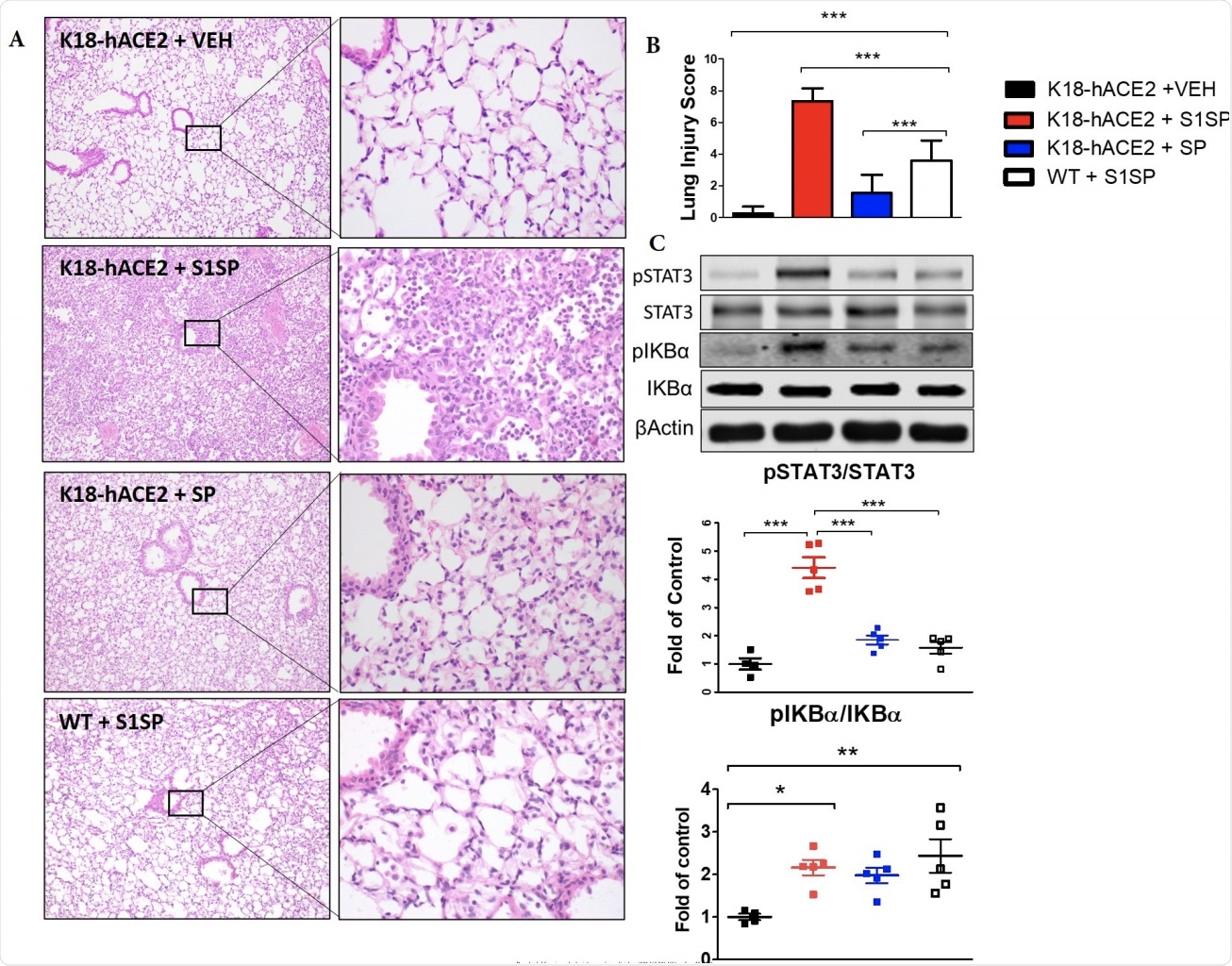 The S1 subunit of the SARS-CoV-2 Spike Protein (S1SP) causes acute lung 378 injury and the activation of the STAT3 and NFκB inflammatory pathways 72 hours after 379 exposure. (A) H&E staining of lung sections demonstrates septal thickening, neutrophil 380 infiltration, and edema in S1SP-instilled K18-hACE2 mice, minimal edema and 381 leucocyte infiltration in S1SP-instilled WT, while SP-instilled K18-hACE2 display minimal 382 changes compared to controls. (B) The Lung Injury Score quantifies maximal injury in 383 S1SP-instilled K18-hACE2 mice, a milder pathology in S1SP-instilled WT and no 384 significant changes in SP-or saline- instilled K18-hACE2 mice. (C) Western blot analysis 385 of lung homogenates revealed increased phosphorylation of IκBα and STAT3. Bands 386 were normalized to β-actin and are shown as fold of control. (Means ± SEM; n=5; *: 387 p<0.05; **: P<0.01; ***: P<0.001 with one-way ANOVA and Tukey’s).
The S1 subunit of the SARS-CoV-2 Spike Protein (S1SP) causes acute lung 378 injury and the activation of the STAT3 and NFκB inflammatory pathways 72 hours after 379 exposure. (A) H&E staining of lung sections demonstrates septal thickening, neutrophil 380 infiltration, and edema in S1SP-instilled K18-hACE2 mice, minimal edema and 381 leucocyte infiltration in S1SP-instilled WT, while SP-instilled K18-hACE2 display minimal 382 changes compared to controls. (B) The Lung Injury Score quantifies maximal injury in 383 S1SP-instilled K18-hACE2 mice, a milder pathology in S1SP-instilled WT and no 384 significant changes in SP-or saline- instilled K18-hACE2 mice. (C) Western blot analysis 385 of lung homogenates revealed increased phosphorylation of IκBα and STAT3. Bands 386 were normalized to β-actin and are shown as fold of control. (Means ± SEM; n=5; *: 387 p<0.05; **: P<0.01; ***: P<0.001 with one-way ANOVA and Tukey’s).
The results of the current study were found to be significant when comparing the control saline and wild-type groups with those that received the S protein, as they presented with either no or minimal manifestations of lung injury. While it is understandable that the saline control group did not exhibit these symptoms, the wild-type mice that received the S protein presented with milder symptoms of COVID-19 as compared to the K18-hACE2 transgenic mice.
The researchers also found a direct effect of the S1 protein on human lung microvascular endothelial cell barrier integrity in culture, with there being a decrease in cultured human pulmonary microvascular transendothelial resistance and barrier function. =
Conclusion
This study provides novel findings of a COVID-19-like response by an essential virus-encoded protein by SARS-CoV-2 in vivo. Moreover, this study shows that SARS-CoV-2 can be lethal in transgenic mice with overexpression of ACE-2 in comparison with the wild-type mice that can present with varied symptoms. The activated or cleaved form of the S protein, which is also known as the S1 protein, can cause strong pulmonary and systemic inflammatory responses in the K18-hACE2 transgenic mice as compared to the control groups.
Utilizing an animal model with the hACE2 receptor can therefore provide a reliable and cost-effective animal model for studying COVID-19. These researchers have provided a premise for the effect of the S1 protein on ALI. In fact, the researchers suggest that the S1 protein can play the role of a ‘toxin,’ which can contribute to the pathogenesis of systemic inflammation when the virus is released.
This study hopefully provides greater insight into potential therapeutic targets through their use of a reliable animal model. In turn, the researchers encourage other scientists to utilize this model to develop novel therapeutics that can reduce COVID-19 mortality rates globally.
Journal reference:
- Biancatelli, R. C., Solopov, P., Sharlow, E., et al. (2021). The SARS-CoV-2 Spike Protein Subunit 1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology. doi:10.1152/ajplung.00223.2021. https://journals.physiology.org/doi/abs/10.1152/ajplung.00223.2021