Peter Nguyen is a Research Scientist at the Wyss Institute at Harvard University and Luis Soenksen is a Research Scientist and Venture Builder at the MIT Jameel Clinic for AI & Healthcare.
Current commercially available wearables (e.g., FitBit or Apple Watch) detect physiological signals electronically. However, they cannot detect exposure to a pathogen or a toxin. That is something that currently requires an entire laboratory to process samples.
Our sensors can now bring that same testing power to wearables to detect and identify pathogens (any bacteria or virus) as well as toxins. In essence, our technology miniaturizes an entire laboratory onto a wearable garment. When the pandemic hit, we immediately pivoted our work to developing a wearable that could detect COVID-19 in an unobtrusive, inexpensive, and quick way.
SARS-CoV-2 Virus. Image Credit: Dotted Yeti/Shutterstock.com
Nearly 4 million people have died so far from the COVID-19 outbreak. Why is it important for researchers and organizations to come together and work collaboratively to help advance our detection and prevention methods?
Collaboration within the research community by sharing data, ideas, and constructive feedback is essential to making sure we have a diversity of approaches and technologies at our disposal. We all build off of each other in a wonderful way, the amazing vaccine technology that arose from this pandemic is an example of that.
Our detection/monitoring technology needs to catch up. We have benefited greatly from thoughtful discussions with researchers in disparate fields and clinicians, too. It’s especially important for a multidisciplinary project such as this.
What are biosensors and how are they able to detect pathogens and toxins?
Biosensors use engineered genetic circuits to create sensors and detectors for a desired molecular target through biology, in contrast to electronic/mechanical/optical sensors. We think biosensors are uniquely suited for pathogen and toxin detection, as they are most able to directly interface with these molecular components.
Our physiological responses to these threats are fundamentally biological in nature. By reengineering the interactions between biological molecules, highly sensitive sensors can be created. Biosensors will enable the next generation of artificially engineered wearables that have sensing capabilities similar to that of our own skin or immune system.
You have been working on this wearable freeze-dried cell-free (wFDCF) technology for over three years and first used it with paper. What problems did you encounter when attempting to recreate this biosensor so that it is wearable and how did you overcome them?
Our work for the COVID-19 face mask is definitely built upon our previous Ebola and Zika paper-based diagnostics. Those tests demonstrated the freeze-dried technology but still required a trained user to prepare a sample, apply it to the paper, and instruments to process it. For our current work, we wanted to focus on having the technology be autonomously operating, with automatic sample collection, integrated sample preparation, and minimal user intervention.
For the face mask, all the user has to do to activate the sensor to begin the analysis is press a button. These various obstacles were not trivial, taking us several years to engineer. A big problem we encountered was evaporation, as the FDCF reactions need water to work. So much of the engineering in the wearables was designing the sensors to minimize evaporation while maintaining flexibility and continuity with the environment, by using specially designed hydrophobic elastomeric sensor chambers.
Also, the face mask sensors used a more complex multi-reaction design, requiring time delays to ensure that each freeze-dried reaction occurred in a stepwise manner and was yet able to run autonomously. Each step and component had to be arduously optimized so that the entire system executed robustly. There was a lot of material optimization as well, to ensure that we had the right flow of water and compatibility with the various reagents.
Please can you describe how you carried out your latest research into developing this wearable biosensor? How does this biosensor work?
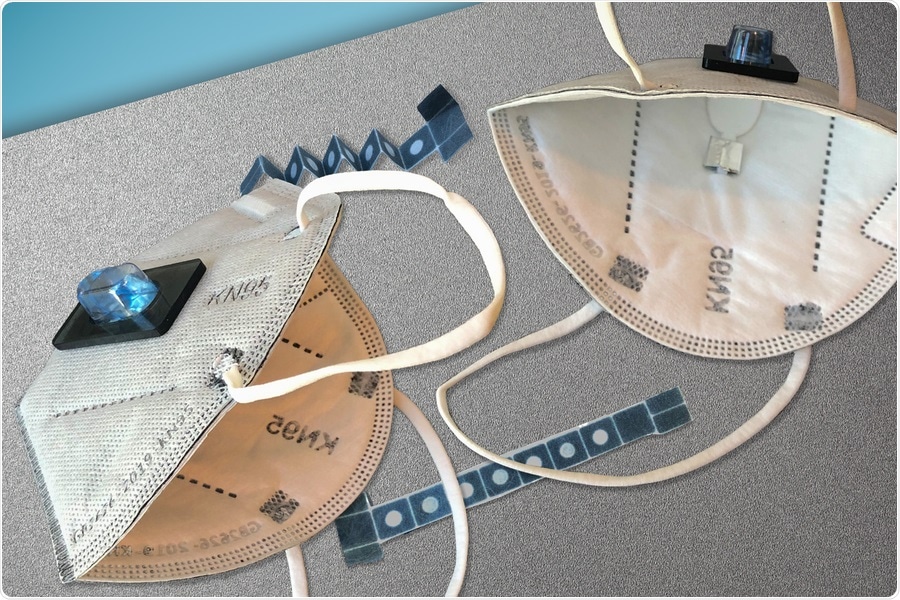
The COVID-19 sensor here also contains freeze-dried biological sensors (based on CRISPR enzymes). Attached to the sensor is a sample collection pad that collects the user’s breath aerosols. When the user has worn the mask for an appropriate amount of time (a minimum of 15-30 minutes), they press a button that pierces a water-filled blister pouch, which immediately wicks water through the sample collection pad, pushing along any viral particles into the sensor for analysis. The sensor contains three freeze-dried reactions, each separated by a dissolvable time delay.
First, an optimized detergent mixture disrupts any viral membranes. The second reaction is a coupled reverse transcriptase – recombinase polymerase amplification (RT-RPA) isothermal reaction, which converts the viral RNA to DNA and amplifies an area of it (the gene coding for the spike protein). The third and last reaction is the CRISPR (Cas12a) sensor, which detects the amplified DNA. There is a probe molecule that gets cleaved (cut) by the Cas12a if it is activated.
At the very end, once the sensor has been executed, the reaction flows to a lateral flow strip (similar to a pregnancy test) where the result is presented simply as a visible pattern of bands that varies by virtue of whether the probe has been cleaved or not.

Face Mask that can detect COVID-19. Image Credit: Wyss Institute for Biologically Inspired Engineering at Harvard University
Your face mask can detect the presence of SARS-CoV-2 with the same accuracy as standard PCR tests. What other advantages does this face mask have over other methods used to diagnose COVID-19 such as PCR tests?
- It’s very convenient – if you are already wearing a mask, why not have a test while you are wearing one?
- It’s noninvasive, detecting the virus from the user’s breath. The longer the breath capture, the more sample available for the analysis.
- It is inexpensive - currently, our prototype COVID-19 diagnostic sensor costs in total approximately $5 USD, not including the cost of the mask itself. At this cost, we think it would be competitive as a disposable all-in-one mask and test. We anticipate that with a mass-manufactured product, this price point should drop.
- It hits the sweet spot of sensitivity and speed – unlike an antigen test, this is a nucleic acid amplification test (NAAT), which directly detects the genome of the virus. Unlike a typical PCR test, this face mask test can give results within 90 minutes without needing to send anything to a lab.
- No instrumentation and minimal user operation – The sensor is fully integrated into the material, and all the user needs to do to activate it is press a button. There are no instruments or power needed.
- It runs at room temperature – This is something that sounds trivial, but it actually quite hard to achieve. All of the reactions are able to work at room temperature, which was what enabled us to dispense with laboratory instruments (for example, heating required by PCR).
What other applications could this face mask potentially be applied to?
One immediate application could be detecting and monitoring the spread of COVID-19 variants. If masks were mailed to a population and the results reported on an app, we might be able to get an extremely detailed map of the spread of a variant, enabling quick policy decisions.
If there was a particularly bad influenza season, these masks could potentially be used to distinguish between the flu and the common cold, enabling rapid administration of antivirals.
There is research showing that other diseases and even cancer can be detected through exhaled volatile organic compounds (VOCs). If we could develop biosensors to detect these VOCs, it could greatly expand the range of what is detectable beyond respiratory pathogens.
Do you believe that this wearable biosensor will help us to further detect coronavirus cases early enabling us to stop the spread of the virus?
One shortcoming in our global preparedness laid bare during this pandemic was the lack of rapid and sensitive testing, which greatly lagged behind the surging infections. Our mask technology could allow us to greatly increase the testing frequency to where it needs to be, enabling potentially daily testing by having a diagnostic that is easy to use for the general population without the need of a laboratory (as the test is completely self-contained and only requires the press of a button).
We believe that by reducing the barrier to diagnostic testing so that it can be performed anywhere by anyone and yet still provide robust results, we could drastically increase the density and frequency of testing. That would be a key piece of the puzzle that is needed to stop this current pandemic in its tracks and contain any future disease outbreaks before it becomes a pandemic.
Face Mask. Image Credit: MIT
What are the next steps in your research?
Given the low cost, the convenience, the shelf stability, and the high performance of the face mask diagnostics, we think manufacturers will be excited to work with us to develop a commercial version from our prototype.
We are actively looking for commercial partners to engage in design for mass manufacturing of the face mask. This process could be quite accelerated with the right partners and government support. We are also looking into developing versions of the face mask sensor that can differentiate between the COVID-19 variants.
Where can readers find more information?
About Dr. Peter Nguyen
Peter Nguyen received a B.S. in Biochemistry and B.A. in Philosophy from the University of Texas, his M.Bs. from the Keck Graduate Institute, and his Ph.D. in Biochemistry from Rice University.
At the Wyss Institute, Peter currently works on programmable probiotics and freeze-dried cell-free manufacturing technology across multiple platforms. His research interests also include engineering systems for the precise self-assembly and control of nanoscale structures; protein engineering for synthetic biology and biomedical applications.
About Dr. Luis R. Soenksen
Luis R. Soenksen is a serial entrepreneur and medical device expert currently acting as MIT’s first Venture Builder in Artificial Intelligence and Healthcare, where he is leading the development and launch of multiple cutting-edge AI ventures with faculty and students across MIT.
Luis holds a Ph.D. in Mechanical Engineering from MIT, as well as a bachelor’s degree in biomedical engineering from the Monterrey Institute of Technology, and a Master’s of Science in Bioengineering from Johns Hopkins University. His training includes medical device design, development, and manufacturing, as well as clinical testing and deployment of healthcare ventures.
In research, Luis has made significant contributions to the fields of bioengineering, bio-design education, tissue engineering, synthetic biology, and artificial intelligence. Luis work has been published in several high-impact journals such as Science and Nature, research that has also led to several patented biomedical technologies and co-founding of 4 start-up companies internationally.