A team of scientists from France has recently demonstrated photosensitive antiviral activity of the natural compound pheophorbide a (Pba) against a broad range of enveloped viruses, including coronaviruses.
The compound has been found to inhibit the entry and reduced the replication of highly pathogenic coronaviruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Middle East respiratory syndrome coronavirus (MERS-CoV).
A detailed description of the antiviral activities of Pba is currently available on the bioRxiv* preprint server.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative pathogen of coronavirus disease 2019 (COVID-19), is an enveloped, positive-sense, single-stranded RNA virus with a genome size of about 30 kb. As of July 13, 2021, the virus has infected more 187 million people and claimed over 4 million lives globally. Although several potential vaccines against COVID-19 have been developed in record time, effective therapeutic interventions to inhibit the viral spread and reduce disease severity are still largely unavailable.
In order to identify effective antiviral medicines against SARS-CoV-2, scientists have focused on bioactive phytochemicals derived from traditional medicinal plants.
In the current study, the scientists have described antiviral properties of a photosensitizer bioactive natural compound pheophorbide a (Pba) against a wide range of enveloped viruses, including SARS-CoV-2, MERS-CoV, HCoV-229E (common cold coronavirus), yellow fever virus (YFV), hepatitis C virus (HCV), and Sindbis virus (SINV).
Extraction of Pheophorbide a
The compound Pba was isolated from the crude extract of Mallotus oppositifolius plant leaves. Specifically, a series of bioguided fractionation experiments were performed to identify the most bioactive plant compound. After isolation and purification, dose-dependent antiviral activities of both extracted plant compound and commercially available Pba were assessed against HCoV-229E. The findings revealed comparable antiviral activities of both natural and commercial Pba.
Antiviral properties of Pheophorbide a
As Pba is a known photosensitizer, its cytotoxic activity was assessed in the presence and absence of light. The findings revealed that in dark conditions, Pba does not affect cell viability even at high micromolar concentrations. However, it exhibits some cytotoxicity when exposed to light. Regarding antiviral activity, Pba exhibited a robust effect against highly pathogenic SARS-CoV-2 and MERS-CoV at non-cytotoxic concentrations.
Regarding mode of action, Pba mediated inhibition of SARS-CoV-2 host cell entry was found to occur both at the cell surface and after virus endocytosis. Moreover, time-of-addition experiments conducted by adding Pba to cells at different timepoints before and after viral challenge revealed that the compound directly acts on the viral particle to inhibit its entry into host cells.
It is now well-established that the entry of human coronaviruses occurs by attachment and subsequent fusion of the viral envelop with the host cell membrane. To determine at which step Pba inhibits the viral entry, a series of in vitro experiments were conducted using HCoV-229E-infected cells.
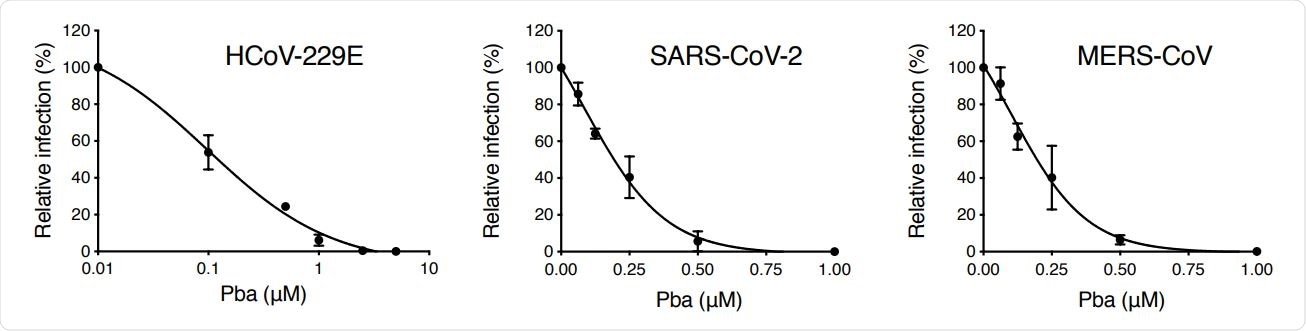
Pba inhibits various HCoVs. Cells were inoculated with HCoV-229E (Huh-7 cells), SARS-CoV-2 (Vero-E6 cells) and MERS-CoV (Huh-7 cells) in presence of various concentrations of Pba. At 1 h p.i, cells were washed and fresh compounds were added to the cells for 9 h (HCoV-229E) or 16 h (SARS-CoV-2 and MERS-CoV) and the supernatants were collected for infectivity titration. Results are expressed as mean ± SEM of 3 experiments.
The findings revealed that Pba inhibits viral entry at the fusion step and not at the attachment step. Further experiments conducted on cells transiently expressing SARS-CoV-2 spike protein revealed that Pba does not directly affect viral spike protein to inhibit virus-cell fusion.
To determine whether the antiviral activity of Pba depends on light exposure, further experiments were conducted on virus-infected cells in the presence or absence of free radical scavengers. The findings revealed that photoactivation of Pba is required for its antiviral activity, which in turn is mediated by the generation of oxygen free radicals. Moreover, the findings revealed that photoactivated Pba inhibits virus-cell fusion by increasing the stiffness/rigidity of the viral envelope. Similar effects of Pba were also observed for several other enveloped viruses, including YFV, HCV, and SINV.
To determine whether in vitro antiviral activities of Pba could be translated in vivo, further experiments were conducted using SARS-CoV-2- or MERS-CoV-infected human primary airway epithelial cells.
The findings revealed that Pba is capable of reducing viral RNA levels and titers in cells even at low micromolar concentrations.
Taken together, the study identifies Pba, which a break-down product of chlorophyll, as a potent phytochemical with broad-spectrum antiviral activity against many enveloped viruses.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Meunier T. 2021. A photoactivable natural product with broad antiviral activity against enveloped viruses including highly pathogenic coronaviruses. bioRxiv, https://doi.org/10.1101/2021.07.09.451770, https://www.biorxiv.org/content/10.1101/2021.07.09.451770v1
- Peer reviewed and published scientific report.
Meunier, Thomas, Lowiese Desmarets, Simon Bordage, Moussa Bamba, Kévin Hervouet, Yves Rouillé, Nathan François, et al. 2022. “A Photoactivable Natural Product with Broad Antiviral Activity against Enveloped Viruses, Including Highly Pathogenic Coronaviruses.” Antimicrobial Agents and Chemotherapy 66 (2). https://doi.org/10.1128/aac.01581-21. https://journals.asm.org/doi/10.1128/AAC.01581-21.