The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused immense suffering and economic hardship to millions of people worldwide. To date, the mechanism of infection by SARS-CoV-2 is still being investigated in an effort to develop new preventative and therapeutic agents.
SARS-CoV-2 entry
Coronaviruses like SARS-CoV-2 often bind to the angiotensin-converting enzyme 2 (ACE2), which is a receptor present on the surface of many types of cells, to enter the host cell. SARS-CoV-2 has a novel substitution on its spike (S) protein, which mediates receptor engagement and viral entry. This K403R substitution contains the Arginine-Glycine-Aspartic acid (RGD) motif, which allows the virus to bind to RGD-binding integrins.
This mutation may promote cell entry and, therefore, play a role in the pathogenicity of SARS-CoV-2. The ACE2 receptor has two sites where integrin binding can occur, one of which includes the RGD domain at 204-206 positions. The second integrin binding location is in the C-terminal end of the cytoplasmic tail of the molecule in the RKKKNKAR sequence.
ACE2 has been reported to bind to integrin β1 in heart failure in humans, which appears to promote RGD-mediated adhesion of cells.
What are integrins?
Integrins are membrane proteins that extend across the cell membrane and are involved in cell adhesion. Integrins have both α and β subunits that can be activated by highly regulated pathways in both directions.
Integrin molecules have three states that interconvert, depending on both their conformation and ligand affinity. The first state is an inactive, bent-closed state (BCS), with little affinity for binding molecules in the extracellular matrix (ECM).
When primed, the integrin molecule shows a second extended-closed state (ECS) with a somewhat higher affinity for binding. Thirdly, the active and extended-open state (EOS) has the highest binding affinity for ECM ligands.
Integrins must interact with the cytoskeleton, which indicates that both cell adhesion and migration are affected. Thus, conformational change in an integrin molecule can cause cellular signals to arise, which can increase ligand binding affinity and avidity, leading to alterations in cytoskeletal arrangement that allow for viral entry.
The integrin-ligand binding is brought about by divalent metal cations such as calcium or magnesium, which interact with the Metal Ion Dependent Adhesion Site (MIDAS) domain. Manganese (Mn) have also been found to interact with the MIDAS domain to stabilize the ECS conformation under test conditions.
Recent research suggests that SARS-CoV-2 could bind to more than one receptor by acting as a central ligand and thus forming a complex of receptor-ligand interactions leading to multiple signals. ACE2 can also do the same thing via its MIDAS domain, which acts the same way.
What did the study find?
In the current study, the scientists used ultraviolet-inactivated SARS-CoV-2 in order to work with the virus outside of biosafety level 3 (BSL3) conditions. These particles were labeled fluorescently by octadecyl rhodamine B (R18), which is a fat-soluble dye that penetrates into the envelope membrane.
The experiment showed that activation by Mn ions sped up viral entry; however, the final number of infected cells was only 20% higher when compared to controls. The scientists also made use of the integrin antagonists BTT 3033, ATN-161, and GLPG0187.
BTT 3033 binds near the MIDAS domain to stabilize the BCS state, whereas ATN-161 is a non-RGD peptide that reduces the infectivity of SARS-CoV-2. Thirdly, GLPG0187 has a broad spectrum of RGD integrin antagonism.
When cells were treated in the presence of Mn ions, 20% more cells were occupied by the virus. This fraction did not respond to treatment with BTT 3033, which does not bind to integrins following their activation by Mn ions.
The other two integrin antagonists produced no difference in binding after co-treatment with Mn ions. However, GLPG0187 competes with the virus more efficiently than ATN-161, thus producing better inhibition of SARS-CoV-2. Evidently, this suggests that SARS-CoV-2 must bind to integrin RGD to enter the cell, and that the integrin must be in the ECS state for this to occur.
Through the use of confocal microscopy to visualize the live virus entering the cell, the researchers found that cells treated with GLPG0187 lost their capability of cell adhesion, probably because integrins could no longer mediate this entry. BTT 3033 treatment reduced virus binding to the cell surface and prevented the inward movement of the virus.
Inhibition of Ga13-integrin interaction blocks SARS-CoV-2R18 cell entry
Integrin activation begins with the binding of a molecule called talin to the cytoplasmic tail. The resulting signal causes the integrin, which is then shifted to the ECS state, to bind to the ligand.
The immobilized ligand causes local resistance to a tensile force applied by the cytoskeleton on the adaptor protein to which the integrin binds. This force stabilizes integrin-ECM binding.
The talin-binding causes resistance that leads to outside-in signaling. In turn, this results in transient replacement of talin by a G protein subunit (Gα13), along with cell spreading, retraction, migration, and receptor internalization.
Cells treated with two myristoylated peptides; namely, mP6 and mP13 peptides, did not allow viral entry. These peptides suppress Gα13-mediated outside-in integrin activation. Therefore, it seems that SARS-CoV-2-integrin binding triggers this activation.
Taken together, the inability to activate integrin following SARS-CoV-2 binding prevents cell infection. Here, mP6 did not show significant inhibition, thereby suggesting that the Ga13-mediated signaling is expendable once infection is established and viral replication has begun. However, mP13 continued to show inhibitory efficacy, thus indicating that talin-induced integrin signaling continues throughout the viral lifecycle.
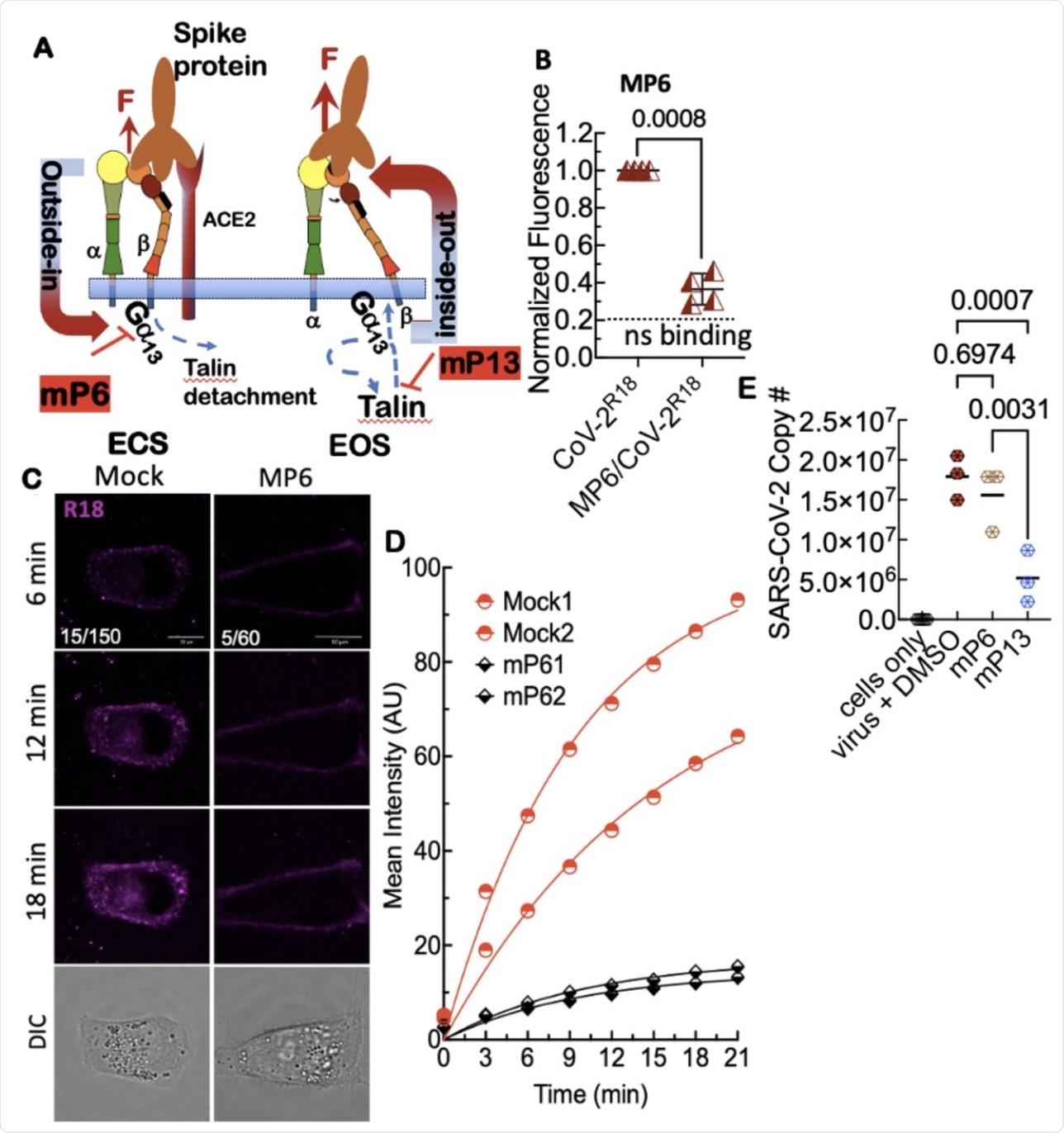 A. Model of outsideinside-out signaling for integrin-mediated cell entry. Hypothetical SARS-CoV-2 binding to integrin β1 initiates Gα13 binding to the β1 cytoplasmic tail, which stimulates outside-in signaling in the absence of a known receptor-stimulated GPCR mediated inside-out signaling. mP6 is a specific inhibitor of Gα13 binding to the β1 cytoplasmic tail. B. Relative fluorescence readings of suspension Vero E6 cells after 30 min incubation with SARS-CoV-2R18 in vehicle and 100 μM mP6 treated cells. C. Live cell imaging of SARS-CoV-2R18 (magenta) binding and endocytosis shows cell membrane and perinuclear localization of SARS-CoV-2R18 vesicles while virus is seen to remain at the plasma membrane in cells treated with 50 μM mP6. LUT ranges shown in the bottom left corner of 6-min timepoint images. Scale bars, 10 μm. D. Traces of absolute intensity values of virus binding over time. Two representative cells for each condition are plotted from data acquired on the same day to enable direct comparison of intensity values. For comparison, mock-treated cell data are the same as in Figure 4. Data were fit to a non-linear regression function with arbitrary constants for appearance purposes. E. Inhibition of SARS-CoV-2 productive infection. Suspension Vero E6 cells were preincubated with 250 μM mP6 and 250 μM mP13 for 30 min and followed by infection with 0.01 MOI SARS-COV-2 for an additional 60 min incubation. Cells were washed twice and transferred to a 12 well plate for 48 hours and assayed for viral RNA by RT-qPCR.
A. Model of outsideinside-out signaling for integrin-mediated cell entry. Hypothetical SARS-CoV-2 binding to integrin β1 initiates Gα13 binding to the β1 cytoplasmic tail, which stimulates outside-in signaling in the absence of a known receptor-stimulated GPCR mediated inside-out signaling. mP6 is a specific inhibitor of Gα13 binding to the β1 cytoplasmic tail. B. Relative fluorescence readings of suspension Vero E6 cells after 30 min incubation with SARS-CoV-2R18 in vehicle and 100 μM mP6 treated cells. C. Live cell imaging of SARS-CoV-2R18 (magenta) binding and endocytosis shows cell membrane and perinuclear localization of SARS-CoV-2R18 vesicles while virus is seen to remain at the plasma membrane in cells treated with 50 μM mP6. LUT ranges shown in the bottom left corner of 6-min timepoint images. Scale bars, 10 μm. D. Traces of absolute intensity values of virus binding over time. Two representative cells for each condition are plotted from data acquired on the same day to enable direct comparison of intensity values. For comparison, mock-treated cell data are the same as in Figure 4. Data were fit to a non-linear regression function with arbitrary constants for appearance purposes. E. Inhibition of SARS-CoV-2 productive infection. Suspension Vero E6 cells were preincubated with 250 μM mP6 and 250 μM mP13 for 30 min and followed by infection with 0.01 MOI SARS-COV-2 for an additional 60 min incubation. Cells were washed twice and transferred to a 12 well plate for 48 hours and assayed for viral RNA by RT-qPCR.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
What are the implications?
The current study demonstrates that integrins are closely linked to SARS-CoV-2 cell entry via endocytosis. Broad-spectrum antagonists of RGD domains suppressed cell entry irrespective of integrin activation status.
However, SARS-CoV-2 binds to BCS integrins, which stabilizes them and promotes its own endocytosis into the host cell. Treatment of the cells with BTT3033, which is an allosteric antagonist of integrin extension, was found to successfully prevent viral entry.
“Thus, our data contextualize integrin extension as the ‘sine qua non of integrin cell adhesion function,’ which in turn is an essential condition for integrin-mediated cell entry by SARS-CoV-2.”
Activated and inactive integrins are treated differently by the cell. Whereas the activated integrin that is bound to SARS-CoV-2 is routed to the perinuclear area by proteins like neuropilin 1 (NRP1), the inactive integrin is instead recycled back via a different adaptor protein to the cell membrane. This could explain how NRP1, which is found at high levels in the olfactory mucosa, is able to mediate viral entry, as it binds to short linear motifs (SLiMs) in the cytoplasmic tails of ACE2 and integrins that mediate endocytosis.
“Our study represents an initial step forward in establishing a mechanistic role for SARS-CoV-2-mediated integrin activation required for cell entry and productive infection.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Simons, P., Rinaldi, D. A., Bondu, V., et al. (2021). Integrin activation is an essential component of SARS-CoV-2 infection. bioRxiv. doi:10.1101/2021.07.20.453118. https://www.biorxiv.org/content/10.1101/2021.07.20.453118v1
- Peer reviewed and published scientific report.
Simons, Peter, Derek A. Rinaldi, Virginie Bondu, Alison M. Kell, Steven Bradfute, Diane S. Lidke, and Tione Buranda. 2021. “Integrin Activation Is an Essential Component of SARS-CoV-2 Infection.” Scientific Reports 11 (1): 20398. https://doi.org/10.1038/s41598-021-99893-7. https://www.nature.com/articles/s41598-021-99893-7.