The development of vaccines that are safe and effective in countering the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus responsible for the coronavirus disease 2019 (COVID-19), is largely considered the only way out of the current pandemic. However, there remain concerns surrounding the efficacy of COVID-19 vaccines in immunocompromised individuals.
 Study: Cell-Mediated and Humoral Immune Response to 2-Dose SARS-CoV-2 mRNA vaccination in Immunocompromised patient population. Image Credit: BaLL LunLa / Shutterstock.com
Study: Cell-Mediated and Humoral Immune Response to 2-Dose SARS-CoV-2 mRNA vaccination in Immunocompromised patient population. Image Credit: BaLL LunLa / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
In the current study, the scientists aimed to define the parameters of cell-mediated and antibody-mediated immune responses following COVID-19 vaccination in immunocompromised patients. Earlier research has indicated that antibody responses are weaker in immunocompromised patients following vaccination with the SARS-CoV-2 vaccine; however, not much is known about the cell-mediated response in this group.
About 1 in 25 Americans have weakened immunity, which makes a significant portion of this nation’s population more vulnerable to severe COVID-19. As a result, immunocompromised individuals have been designated as a priority group for vaccination efforts.
The current study included 16 immunocompetent healthy healthcare workers who were tested at a median of 17 days after the second dose of BNT162b2 messenger ribonucleic acid (mRNA) vaccine, as well as 100 immunocompromised patients who had also received two doses of either the BNT162b2 or Moderna vaccine, which is also an mRNA vaccine.
The latter group contained those who had a history of organ transplant, blood cancer, autoimmune disease for which they were taking antimetabolites, or primary immunodeficiency.
What were the findings?
All healthy controls developed a positive interferon-γ release assay (IGRA), which indicates a normal T cell response to the vaccine antigen, whereas only about 70% of immunocompromised patients exhibited the same results. The median interferon-γ levels were similar in both groups.
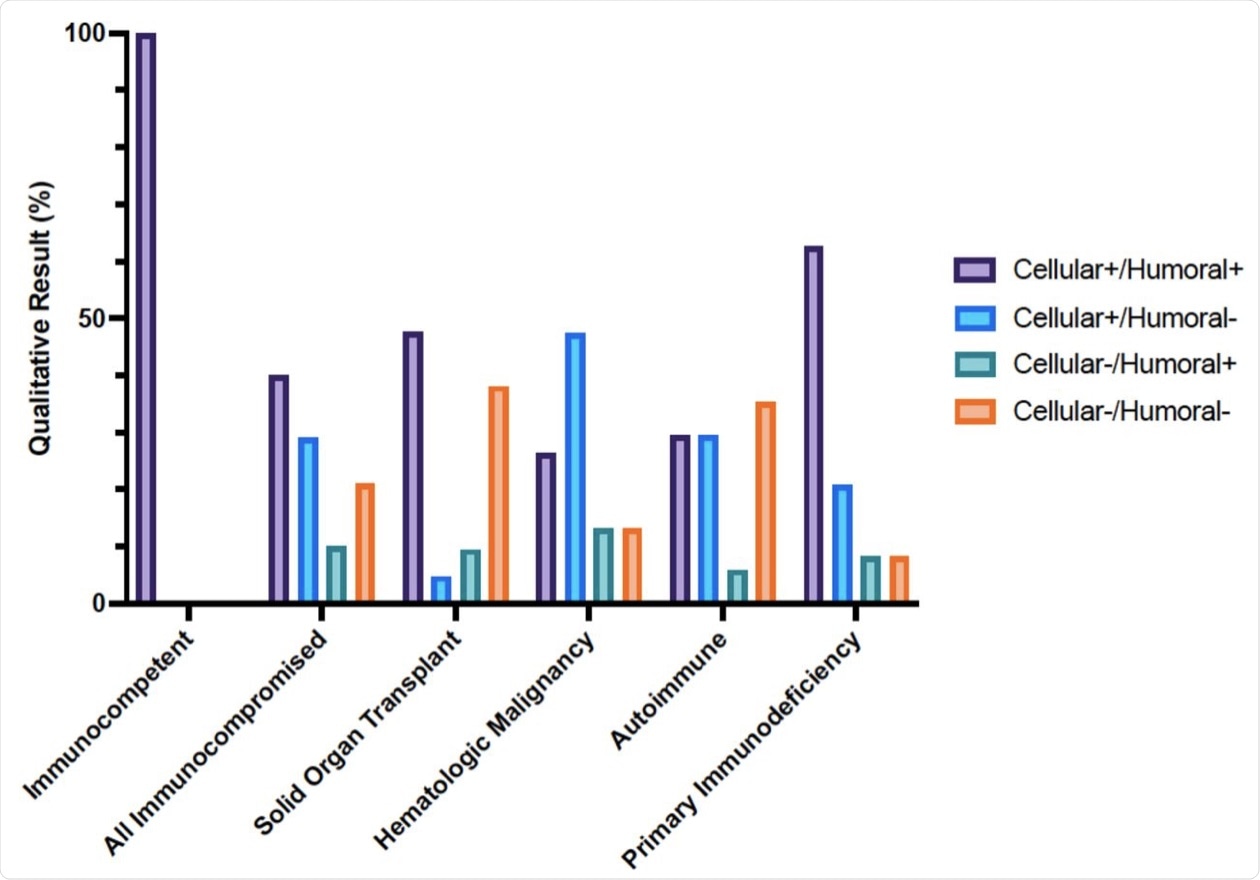 Cellular and humoral immune responses to SARS-CoV-2 mRNA vaccine in immunocompetent and immunocompromised patients determined with clinical assays. Percentage of immunocompetent and immunocompromised patients with positive or negative SARS-CoV2 interferon-γ release assay and Anti-SARS-CoV-2 S1 IgG ELISA result after 2 doses of SARS-CoV2 mRNA vaccination.
Cellular and humoral immune responses to SARS-CoV-2 mRNA vaccine in immunocompetent and immunocompromised patients determined with clinical assays. Percentage of immunocompetent and immunocompromised patients with positive or negative SARS-CoV2 interferon-γ release assay and Anti-SARS-CoV-2 S1 IgG ELISA result after 2 doses of SARS-CoV2 mRNA vaccination.
The detection of antibodies to the vaccine antigen was also positive in all immunocompetent subjects, which was comparable to only 50% of the immunocompromised group. Notably, the former group also had higher antibody immunoglobulin G (IgG) levels as compared to the immunocompromised group.
In 29% of the immunocompromised participants, the cell-mediated response was not accompanied by a detectable antibody response. Additionally, 10% of the patients in this group had IgG levels as their only positive immune response to the vaccine.
Among immunocompromised patients, both vaccines had a comparable response. However, those under the age of 50 had five times higher IgG levels as compared to older patients, all the while maintaining comparable antibody responses.
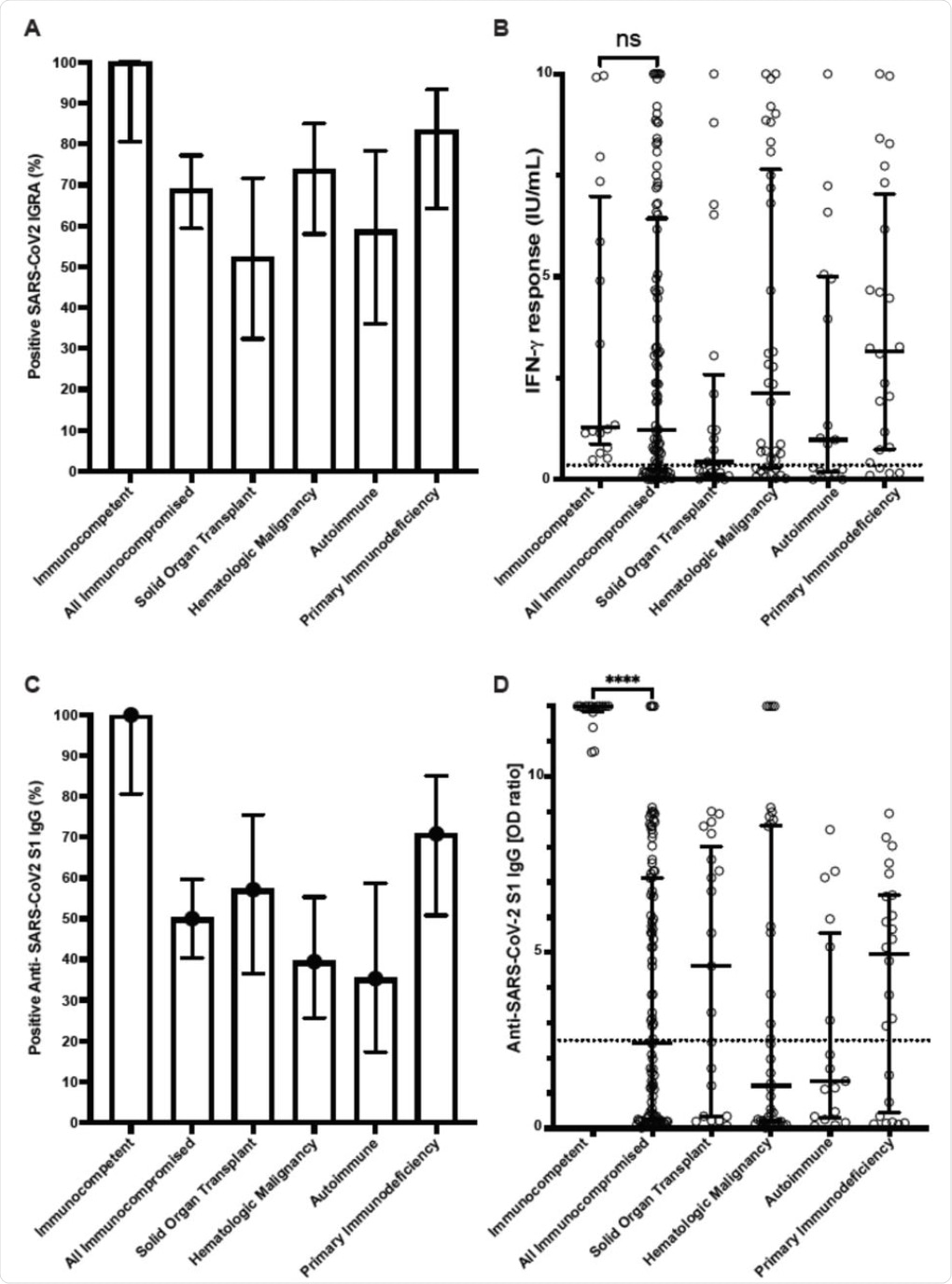 Cellular and humoral immune responses to SARS-CoV-2 mRNA vaccine in immunocompetent individuals and immunocompromised patients determined with clinical assays. (A) Percentage of patients with positive SARS-CoV2 interferon-γ release assay (IGRA) result. (B) Interferon-γ response from IGRA in immunocompetent individuals (n=16) and immunocompromised patients (n=100) (C) Percentage of patients with positive Anti-SARS-CoV2 S1 IgG ELISA result. (D) Anti-SARS-CoV2 S1 IgG optical density (OD) ratio from ELISA performed in immunocompetent and immunocompromised patients. Results for immunocompromised subgroups are also shown. Dotted lines represent the assay cutoffs (0.35 IU/mL for IGRA and 2.5 optical density ratio for IgG). Long solid lines show median, short solid lines show 95% confidence interval. ns, not significant and ****, p<0.0001 by Mann-Whitney U test.
Cellular and humoral immune responses to SARS-CoV-2 mRNA vaccine in immunocompetent individuals and immunocompromised patients determined with clinical assays. (A) Percentage of patients with positive SARS-CoV2 interferon-γ release assay (IGRA) result. (B) Interferon-γ response from IGRA in immunocompetent individuals (n=16) and immunocompromised patients (n=100) (C) Percentage of patients with positive Anti-SARS-CoV2 S1 IgG ELISA result. (D) Anti-SARS-CoV2 S1 IgG optical density (OD) ratio from ELISA performed in immunocompetent and immunocompromised patients. Results for immunocompromised subgroups are also shown. Dotted lines represent the assay cutoffs (0.35 IU/mL for IGRA and 2.5 optical density ratio for IgG). Long solid lines show median, short solid lines show 95% confidence interval. ns, not significant and ****, p<0.0001 by Mann-Whitney U test.
What are the implications?
The current study shows that vaccines are less likely to elicit a humoral response against SARS-CoV-2 than a cell-mediated response. Conversely, no difference was found in the interferon-γ levels between the two groups.
A noticeable difference was reported in the response by either the humoral or cell-mediated immune responses between organ transplant recipients and those with autoimmune disease, with 35% to 38% failing to show one of these types of immunity, respectively.
Taken together, the findings reported here imply that the immune response to the COVID-19 vaccine must be monitored in immunocompromised patients, especially those who have received organ transplants, severe autoimmune disease, or who are older than 50. The use of IGRA or serological assays is recommended for this purpose.
By monitoring these patients closely, healthcare providers will be able to identify patients who may benefit from booster vaccines to increase their antibody and/or cell-mediated immunity, or from prophylactic monoclonal antibody treatment.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Ramanathan, M., Murugesan, K., Yang, L. M., et al. (2021). Cell-Mediated and Humoral Immune Response to 2-Dose SARS-CoV-2 mRNA vaccination in Immunocompromised patient population. medRxiv. doi:10.1101/2021.07.21.21260921. https://www.medrxiv.org/content/10.1101/2021.07.21.21260921v1
- Peer reviewed and published scientific report.
Yang, Lu M., Cristina Costales, Muthukumar Ramanathan, Philip L. Bulterys, Kanagavel Murugesan, Joseph Schroers-Martin, Ash A. Alizadeh, et al. 2022. “Cellular and Humoral Immune Response to SARS-CoV-2 Vaccination and Booster Dose in Immunosuppressed Patients: An Observational Cohort Study.” Journal of Clinical Virology 153 (August): 105217. https://doi.org/10.1016/j.jcv.2022.105217. https://www.sciencedirect.com/science/article/pii/S1386653222001500.