With the onset of coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), scientists began an intensive effort to develop vaccines against the virus. These efforts were largely fueled by predictions that public health interventions would inevitably cripple the global economy if continued indefinitely.
In the United States, the first vaccine to receive emergency use authorization was the Pfizer/BioNTech messenger ribonucleic acid (mRNA) vaccine, with the Moderna mRNA vaccine approved shortly after. By February 26, 2021, the Johnson & Johnson (J&J)/Janssen human adenovirus (Ad26) vaccine was also approved in the U.S.. As of July 27, 2021, 188 million people have received at least one dose in the U.S., of which 191 and 138 million doses have been provided by Pfizer and Moderna, respectively.
 Study: Comparative profiles of SARS-CoV-2 Spike-specific milk antibodies elicited by COVID-19 vaccines currently authorized in the USA. Image Credit: Ahmet Misirligul / Shutterstock.com
Study: Comparative profiles of SARS-CoV-2 Spike-specific milk antibodies elicited by COVID-19 vaccines currently authorized in the USA. Image Credit: Ahmet Misirligul / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
COVID-19 in children
An interesting new paper examines how these vaccines can help young patient populations to withstand the virus. Natural infection or vaccination of the mother causes SARS-CoV-2 antibodies to be secreted in the breast milk, thus providing passive immunity to the infant.
Whereas young children and infants are not eligible for COVID-19 vaccination in many countries, about 10% of patients in this age group who are infected with SARS-CoV-2 will experience symptoms that are serious enough to require intensive care. While rare, some young COVID-19 patients can develop a sequel called Multisystem Inflammatory Syndrome in Children (MIS-C), which can be potentially deadly.
In addition to the risk of MIS-C, some children who have recovered from COVID-19 have reported long-haul symptoms, even after the resolution of the infection. Aside from the risks posed to the patients themselves, this group may also transmit the virus to others while asymptomatic.
Taken together, these factors support the need to immunize both young children and infants against COVID-19.
Antibodies in milk
Human breast milk contains a total immunoglobulin (Ig) concentration of about 0.6mg/mL, mostly in the form of IgA (90%). IgA found within breast milk is almost always secretory IgA, whereas secretory IgM comprises about 8%.
The remaining 2% of breastmilk Ig is IgG that primarily originates from the serum. Both secretory IgA and IgM are from the gut-associated lymphoid tissue (GALT), with some derived from B cells in mucosal-associated lymphoid tissue (MALT).
Natural infection with SARS-CoV-2 causes predominantly secretory IgA to be present in the breastmilk. Comparably, at 14 days from the second dose, breast milk appears to be primarily rich in IgG. Though the first vaccine dose elicits a typically IgA-predominant response in milk, the second dose fails to produce this type of antibody.
How was the study conducted?
The current study, which was a follow-up to a previous paper, compares the antibody composition in breast milk following vaccination with any of the three vaccines currently available in the United States. The examination was carried out on samples from 50 different lactating mothers, collected one week before, and 14 or 28 days after the second dose of vaccine, with the mRNA and J&J vaccines, respectively.
None of the subjects had a history of SARS-CoV-2 infection, nor did any milk sample contain SARS-CoV-2-specific IgA before vaccination.
What were the findings?
The findings show that the average specific milk IgG levels were similar between the mRNA vaccines, but were higher than those obtained with the J&J vaccine.
Following receipt of the mRNA vaccines, all samples were found to contain IgG antibodies specific to the SARS-CoV-2 spike protein. With the J&J vaccine, this probability was about 40% less. The IgG titers in samples that were specific for spike-specific antibodies were also significantly reduced relative to breast milk samples obtained from Moderna vaccine recipients.
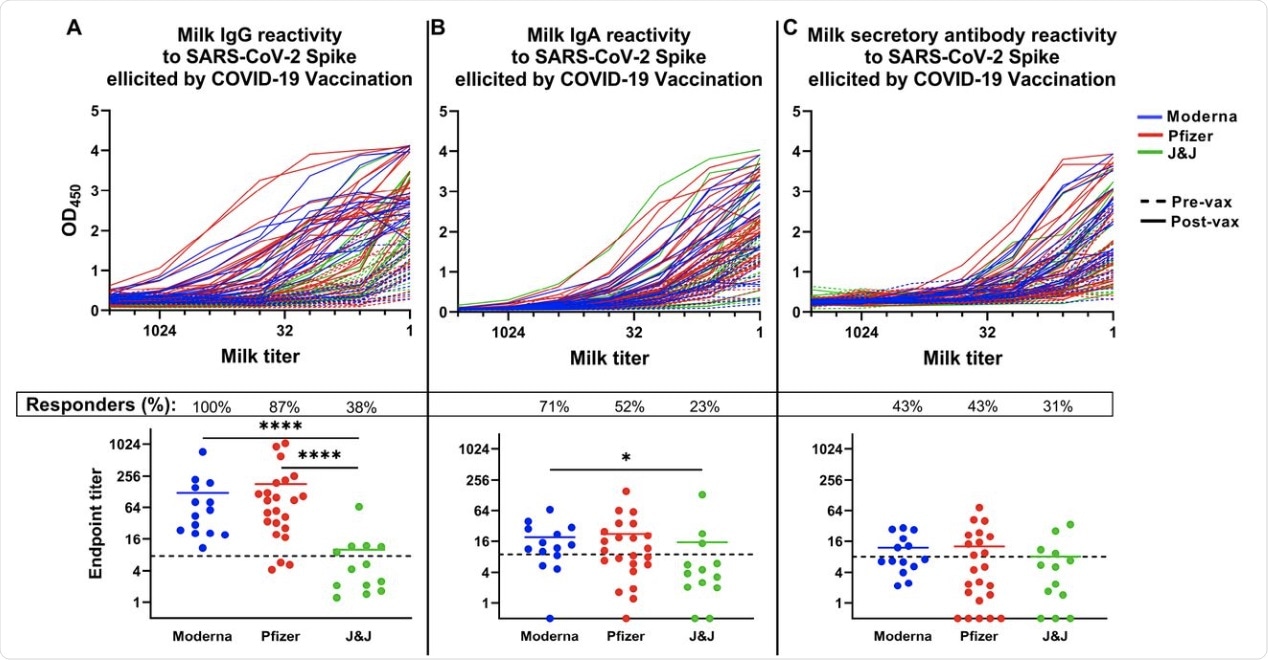 The COVID-19 vaccine-induced SARS-CoV-2 Spike-specific milk antibody response. Plates were coated with full-length recombinant trimeric SARS-CoV-2 Spike. Milk was titrated and added to the plate. Plates were washed and blocked and incubated with appropriate secondary antibody. Titration curves were plotted and used to calculated endpoint titers. (A) IgG titration curves (top panel) and endpoint titers (bottom panel). (B) IgA titration curves (top panel) and endpoint titers (bottom panel). (C) Secretory antibody titration curves (top panel) and endpoint titers (bottom panel). Dotted lines indicate positive cutoff values. Responders (%): Percent of participants receiving this vaccine type exhibiting positive endpoint titers. ****p<0.0001; *0.009<p<0.05. Mean values are shown in scatter plots.
The COVID-19 vaccine-induced SARS-CoV-2 Spike-specific milk antibody response. Plates were coated with full-length recombinant trimeric SARS-CoV-2 Spike. Milk was titrated and added to the plate. Plates were washed and blocked and incubated with appropriate secondary antibody. Titration curves were plotted and used to calculated endpoint titers. (A) IgG titration curves (top panel) and endpoint titers (bottom panel). (B) IgA titration curves (top panel) and endpoint titers (bottom panel). (C) Secretory antibody titration curves (top panel) and endpoint titers (bottom panel). Dotted lines indicate positive cutoff values. Responders (%): Percent of participants receiving this vaccine type exhibiting positive endpoint titers. ****p<0.0001; *0.009<p<0.05. Mean values are shown in scatter plots.
About 70% and 50% of samples from Moderna and Pfizer vaccine recipients, respectively, had spike-targeting IgA in the milk, with not much difference in titer values. Moreover, the Moderna vaccine elicited a higher increase in secretory IgA at 93%, which is comparable to about 74% and 54% with the Pfizer and J&J vaccines, respectively. Overall, the level of secretory antibodies induced by vaccination was low with all vaccines, whether measured in absolute terms or compared to post-infection titers.
Among the three vaccines, about a quarter of Moderna vaccine recipients had a higher titer value as compared to the other two vaccines. While 86% of Moderna recipients showed an increase in the antibody titer values, Pfizer and J&J recipients showed a smaller increase of 61%.
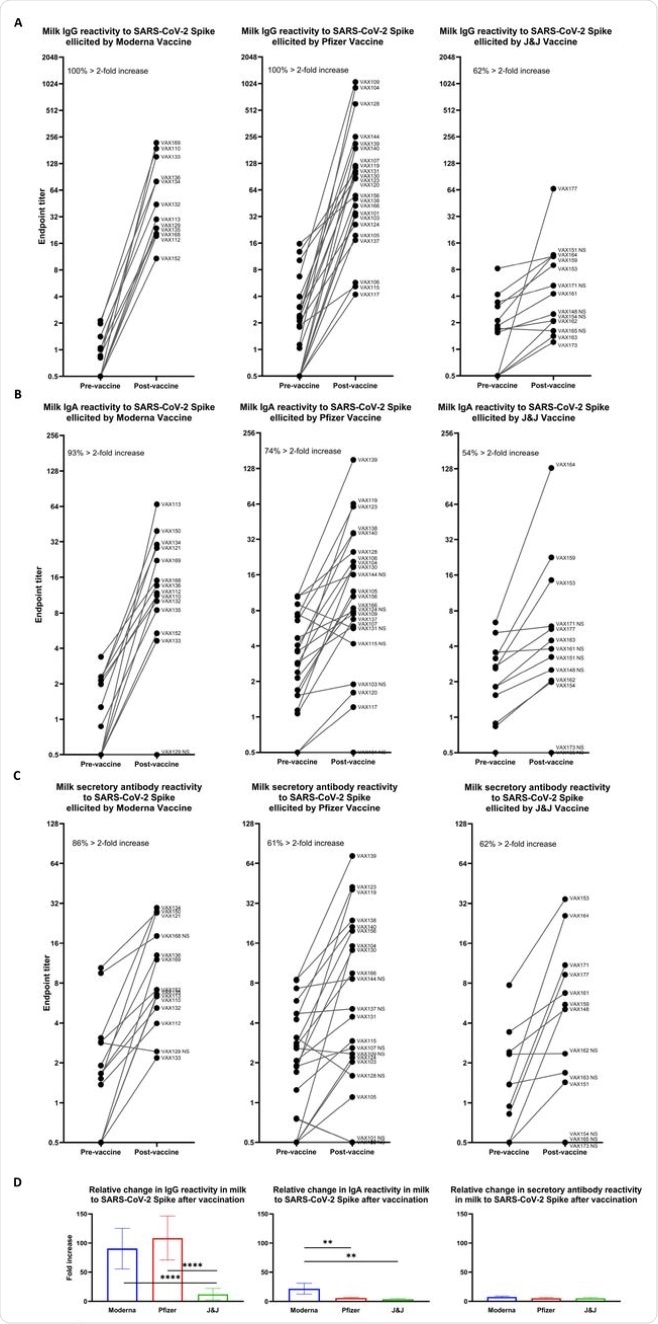 Relative increases in milk antibody reactivity to SARS-CoV-2 Spike elicited by COVID-19 vaccination. Endpoint titers were determined as in Fig. 1 for pairs of milk samples obtained from participants within 1 week before and 14 (Pfizer/Moderna) or 28 (J&J) days after completion of a vaccine regimen. Pre-vaccine and post-vaccine endpoint titers are shown for: (A) IgG reactivity. (B) IgA reactivity. (C) Secretory antibody reactivity. Left panel, Moderna vaccine; center panel, Pfizer vaccine; right panel, J&J vaccine. Each plot indicates the percent of vaccine recipients exhibiting > 2-fold increase in specific endpoint titer between pre- and post-vaccine milk samples. (D) Fold increases in endpoint titers are shown for each vaccine group as indicated. ****p<0.0001; **0.0001<p<0.009. Mean values with SEM are shown.
Relative increases in milk antibody reactivity to SARS-CoV-2 Spike elicited by COVID-19 vaccination. Endpoint titers were determined as in Fig. 1 for pairs of milk samples obtained from participants within 1 week before and 14 (Pfizer/Moderna) or 28 (J&J) days after completion of a vaccine regimen. Pre-vaccine and post-vaccine endpoint titers are shown for: (A) IgG reactivity. (B) IgA reactivity. (C) Secretory antibody reactivity. Left panel, Moderna vaccine; center panel, Pfizer vaccine; right panel, J&J vaccine. Each plot indicates the percent of vaccine recipients exhibiting > 2-fold increase in specific endpoint titer between pre- and post-vaccine milk samples. (D) Fold increases in endpoint titers are shown for each vaccine group as indicated. ****p<0.0001; **0.0001<p<0.009. Mean values with SEM are shown.
What are the implications?
“ The J&J vaccine poorly elicits spike-specific Ab in milk compared to mRNA-based vaccines and that this vaccine should be considered a last choice for immunizing those intending to elicit a strong Ab response in their milk.”
The breast milk antibody response may be modestly enhanced by the Moderna vaccine in terms of secretory IgA titers as compared to the other vaccines. This underlines the need to develop more effective vaccines for use in pregnancy and lactation with the aim of providing the best levels of protection to the breastfeeding infant.
“In areas where COVID-19 vaccines are freely available, in terms of potential passive immunization of infants and young children via breastfeeding or milk sharing, these data suggest that Moderna vaccine is the most ideal, while J&J vaccine should be considered a last resort.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Fox, A., DeCarlo, C., Yang, X., et al. (2021). Comparative profiles of SARS-CoV-2 Spike-specific milk antibodies elicited by COVID-19 vaccines currently authorized in the USA. medRxiv. doi:10.1101/2021.07.19.21260794. https://www.medrxiv.org/content/10.1101/2021.07.19.21260794v1
- Peer reviewed and published scientific report.
Yang, Xiaoqi, Alisa Fox, Claire DeCarlo, Caroline Norris, Samantha Griffin, Sophie Wedekind, James M. Flanagan, Natalie Shenker, and Rebecca L. Powell. 2022. “Comparative Profiles of SARS-CoV-2 Spike-Specific Human Milk Antibodies Elicited by MRNA- and Adenovirus-Based COVID-19 Vaccines.” Breastfeeding Medicine 17 (8): 638–46. https://doi.org/10.1089/bfm.2022.0019. https://www.liebertpub.com/doi/10.1089/bfm.2022.0019.