Several coronavirus disease 2019 (COVID-19) vaccines have received emergency use authorization (EUA) from various regulatory bodies around the world.
Of these includes the messenger ribonucleic acid (mRNA) vaccine BNT162b2 that was developed by BioNTech/Pfizer. The BNT162b2 vaccine can effectively protect humans from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the causal agent of COVID-19. Both clinical trials, as well as real-world conditions, have revealed that two doses of this vaccine are capable of preventing the infection and severe symptoms of COVID-19.
 Study: Correlation of SARS-CoV-2 Breakthrough Infections to Time-from-vaccine; Preliminary Study. Image Credit: Ju Jae-Young / Shutterstock.com
Study: Correlation of SARS-CoV-2 Breakthrough Infections to Time-from-vaccine; Preliminary Study. Image Credit: Ju Jae-Young / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Scientists are extremely concerned about the continual emergence of the SARS-CoV-2 variants. Several of these variants have been classified as Variants of Concern (VOC), owing to their superior infectivity and mortality rates as compared to the original SARS-CoV-2 strain.
Several studies have been conducted to evaluate the vaccine-induced immune responses; however, there remains a gap in research related to the correlation between antibody dynamics and clinical protection against SARS-CoV-2. In the context of VOCs, few studies have successfully differentiated time-from-vaccine and vaccine effectiveness across different strains.
Are vaccines effective against the SARS-CoV-2 Delta variant?
The COVID-19 vaccination rate in Israel has been significantly high. Despite the high vaccination rate, the Delta (B.1.617.2) variant has become the dominant circulating strain in Israel.
This surge in infection rates, even among individuals who are vaccinated, has raised concerns on the efficacy of COVID-19 vaccines against the SARS-CoV-2 Delta variant. However, this scenario contradicts several studies that have reported only a minor difference in the effectiveness of the approved vaccines against the SARS-CoV-2 Delta variant in comparison to the original strain for which the vaccine had been developed.
A new study
Owing to the rapid mass vaccination campaign in Israel, scientists were able to establish the correlation between time-from-vaccine and vaccine effectiveness against the Delta variant. A new retrospective study published on the preprint server medRxiv* compares the incidence rates of breakthrough infections between different time points at which individuals were vaccinated.
The researchers of this study have obtained data from the Maccabi Healthcare Services (MHS). MHS contains data on 2.5 million individuals, which reflects 25% of Israel’s population. The authors considered MHS members who are of or above 16 years old and had received the second vaccine dose of the COVID-19 vaccine between January and April 2021.
Following the guidelines set by the United States Centers for Disease Control and Prevention (CDC), the researchers considered individuals as fully vaccinated if they received two doses of the BNT162b2 vaccine and had received the second dose 21-28 days prior to when the data was collected.
Individuals who were SARS-CoV-2 positive, according to the polymerase chain reaction (PCR) assay test before the start of the study period, were excluded from this study. Two logistic regression models were estimated to correlate the time-from-vaccine and vaccine-induced protection against breakthrough infection.
The regression models
In the first model, the researchers grouped individuals into two separate sets that included early vaccinees and late vaccinees to assess the time-from-vaccine. The early vaccinees group included individuals who received the second dose of the vaccine between January and February 2021, whereas the late vaccinees group consisted of individuals who received the second dose between March and April 2021.
Subsequently, the researchers matched each early vaccinee to a late vaccinee in a 1:1 ratio based on their age group, gender, city of residence, and socioeconomic status. This is because vaccination campaigns prioritized high-risk individuals such as older age groups, people with comorbidities, and front-line workers, as they are extremely prone to get infected.
The second model analyzed the effectiveness of the vaccine based on the time-from-vaccination by comparing individuals belonging to six distinct groups. The groups were constructed based on the month at which an individual was considered to be fully vaccinated. Similar to the earlier model, the same matching and adjustment methods were used.
Study findings
A total of 1,151 individuals were identified as early vaccinees and 760 individuals were recognized as late vaccinees.
In the first model, researchers adjusted comorbidities and reported that 53% of early vaccinees had an increased risk of breakthrough infection. Similarly, model two also showed a higher risk of breakthrough infections in individuals who received vaccines in the earlier months as compared to the individuals who were vaccinated in later months.
Therefore, this study of a large cohort of MHS members who were vaccinated with the BioNTech/Pfizer vaccine revealed a significant correlation between the time-from-vaccine and the vaccine-induced protection against SARS-CoV-2 infection. Further, this study showed that the risk of infection in early vaccinees was significantly higher as compared to late vaccinees.
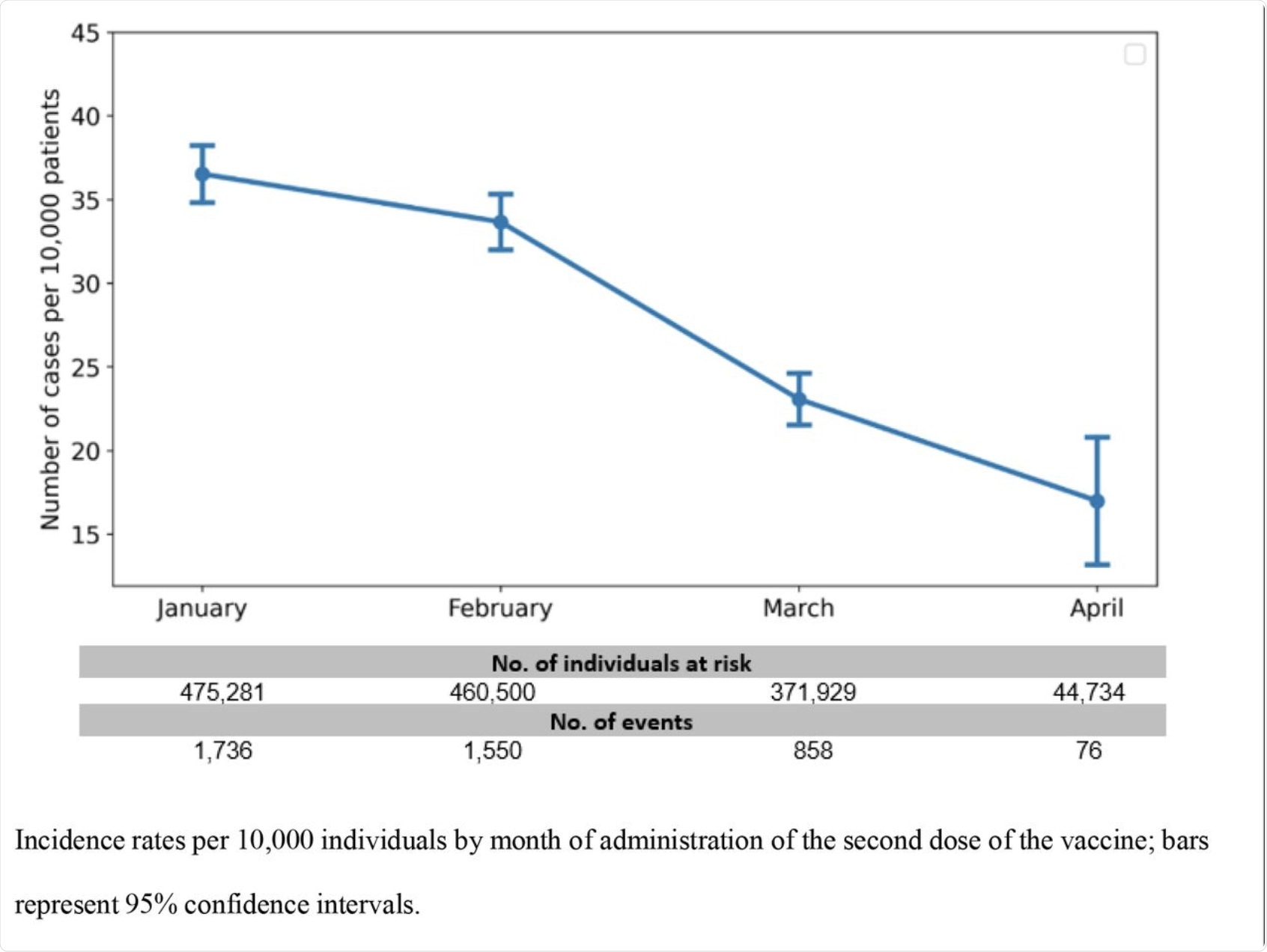 Incidence rate by month.
Incidence rate by month.
Limitations
One limitation of this study is that it is based on only one variant, as the Delta variant was the dominant strain when the study was conducted. The long-term effectiveness of the vaccine against other strains could, therefore, not be determined. Another limitation is that the study did not assess the effect of vaccination time on symptomatic infection or severe COVID-19 disease.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Mizrahi, B., Lotan, R., Kalkstein, N., et al. (2021). Correlation of SARS-CoV-2 Breakthrough Infections to Time-from-vaccine; Preliminary Study. medRxiv. doi:10.1101/2021.07.29.21261317. https://www.medrxiv.org/content/10.1101/2021.07.29.21261317v1.full-text.
- Peer reviewed and published scientific report.
Mizrahi, Barak, Roni Lotan, Nir Kalkstein, Asaf Peretz, Galit Perez, Amir Ben-Tov, Gabriel Chodick, Sivan Gazit, and Tal Patalon. 2021. “Correlation of SARS-CoV-2-Breakthrough Infections to Time-From-Vaccine.” Nature Communications 12 (1). https://doi.org/10.1038/s41467-021-26672-3. https://www.nature.com/articles/s41467-021-26672-3.