The emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus responsible for the coronavirus disease 2019 (COVID-19) has infected over 221 million and caused the deaths of over 4.57 million as of September 6, 2021. Vaccination against COVID-19 is largely believed to be the only effective method to end the current pandemic.
Since December 8, 2020, three different vaccines have been given emergency use authorization by the United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA). These three vaccines include the Pfizer/BioNTech BNT162b2 messenger ribonucleic acid (mRNA), Oxford/AstraZeneca ChAdOx1 nCoV-19 adenoviral AZD1222, and Moderna mRNA-1273 vaccines.
Prior to their approval, clinical trials found that the BNT162b2 and ChAdOx1-S vaccines were highly effective when a two-dose schedule with an interval of three and four weeks was used, respectively. Whereas a single dose of either BNT162b2 or AZD1222 provided 50-70% percent protection against infection and mild disease, they also demonstrated 75-80% protection against hospitalization and death.
Comparatively, after two doses, the level of effectiveness increased to 65-90% against mild disease and 90-100% against severe disease. These levels of vaccine effectiveness (VE) were mostly found in older adults, while effectiveness for individuals belonging to clinical risk groups was not yet determined.
Vaccine efficacy in at-risk groups
Along with age, the presence of other clinical comorbidities can lead to an increased risk of severe COVID-19 disease. Diseases such as diabetes, asthma, chronic liver disease, chronic heart disease, chronic kidney disease, neurological diseases, and diseases associated with immunosuppression have all been linked to a greater risk of hospitalization and death.
Studies on the antibody response to vaccination in individuals with such comorbidities have shown reduced seroconversion rates and antibody responses. However, the mechanisms responsible for these differences in antibody response and their relationship with vaccine effectiveness remain unclear.
In a recent preprint study, the researchers utilized electronic health records (EHR) from a cohort of general practice patients. These records included the sentinel antibody test results. This allowed for the estimation of vaccine effectiveness and antibody response against COVID-19 in patients of different clinical risk groups.
About the study
The current study involved pseudonymized data extracted from computerized medical record (CMR) data that was collected from Oxford-Royal College of General Practitioners Research and Surveillance Centre (RSC).
National COVID-19 testing results are posted electronically in the general practice EHR, from where individual records can be collected. Swab and sera samples were also collected from individuals who exhibited flu-like symptoms.
Two assays were performed with the collected samples, which included anti-SARS-CoV-2 spike (S) and nucleoprotein (N) assays. The N assay helps to determine the presence of antibodies due to natural infection, whereas the S assay helps to determine the presence of both post-infection and vaccine-induced antibodies.
Study findings
In the current study, vaccine effectiveness was determined from a cohort of 5,642,687 people, of which 1,276,517 were above the age of 65 and 1,054,510 were classified as at-risk for COVID-19. A higher proportion of individuals with comorbidities like chronic heart disease and vascular disease (CHD), chronic kidney disease, as well as chronic respiratory and neurological risk groups were vaccinated between December 2020 and January 2021. Comparatively, individuals over the age of 65 were largely vaccinated between January and February 2021, with both groups receiving a relatively equal among of AstraZeneca and Pfizer vaccines.
For individuals who did not belong to a risk group, the seropositivity was 9.0%. However, seropositivity was lower in individuals who were considered at-risk for COVID-19. Aside from the immunocompromised group and those with diabetes, this difference in seropositivity was not significantly different.
Reduced S antibody titers were found in immunosuppressed individuals, as well as those who were morbidly obese, had diabetes, or were included in the CHD risk group, after the first dose of the vaccine. However, this titer increased after the second dose of the vaccine.
Overall, the VE after a single dose of either vaccine was approximately 60%. This VE estimate was not found to vary significantly between each age group.
Comparatively, after two doses of the AstraZeneca and Pfizer vaccines, more notable differences were identified between the age groups. Whereas those between the ages of 16 and 64 had a cohort VE of 93.3% following full vaccination of the Pfizer vaccine, this value was 78% with both doses of the AstraZeneca vaccine. Within the 65 and older age group, full vaccination with the Pfizer and AstraZeneca vaccines achieved VE of 86.7% and 76.$%, respectively.
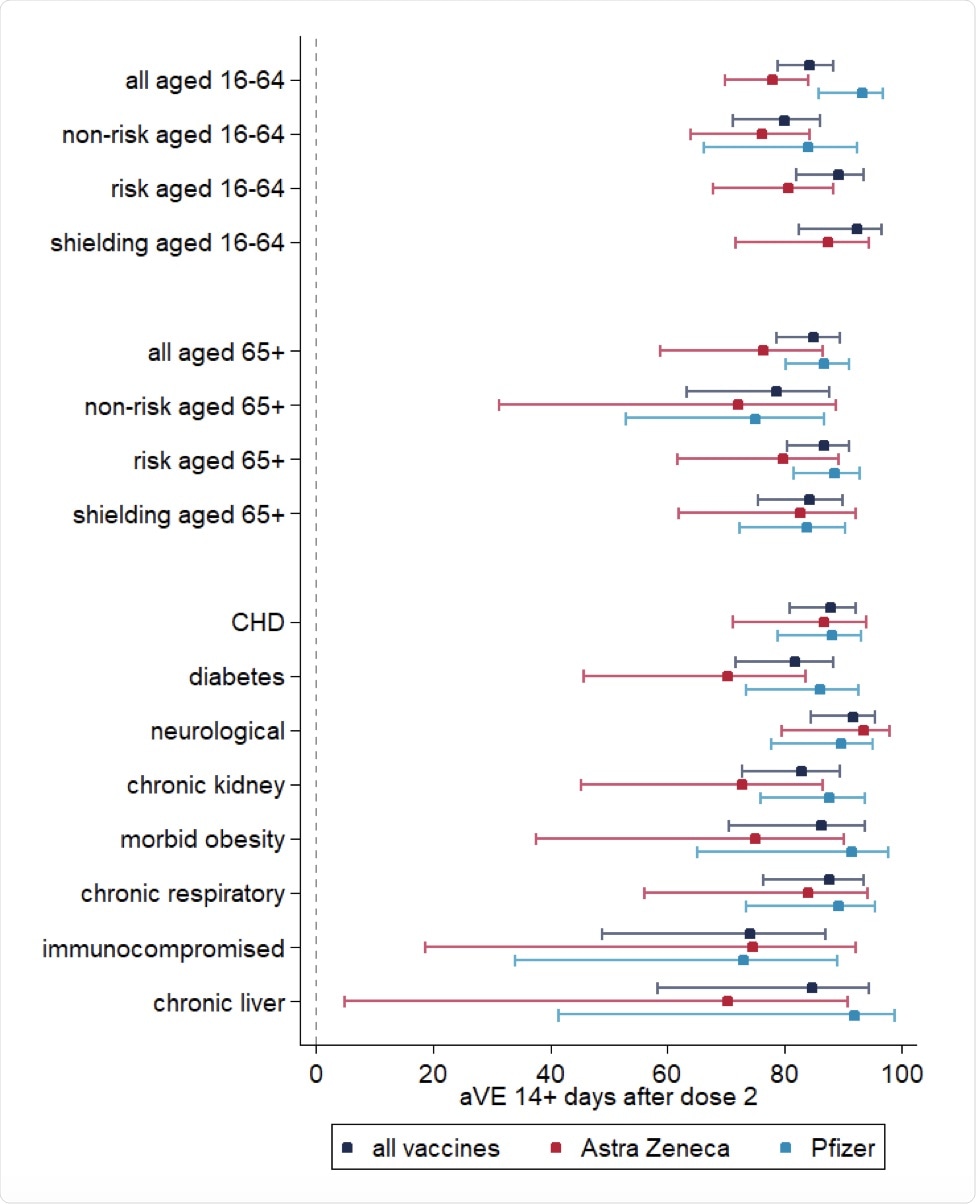
Cohort vaccine effectiveness 14+ days after dose 2 of vaccination.
Conclusions
Taken together, the current study found that a strong S-antibody response occurs following complete vaccination with two different COVID-19 vaccines. Additionally, both of these vaccines were found to be highly effective in preventing symptomatic medically-attended disease, particularly among high-risk patients.
Overall, the findings of the current study agree with immunogenicity and VE findings that have been reported previously. While the data discussed here supports the two-dose vaccine schedule among immunocompromised individuals, the authors discuss the potential advantages associated with reducing the interval between doses in this patient population.