From studies conducted on animals, evidence has emerged that both neutralizing activity and Fc-mediated effector functions of neutralizing antibodies provide some levels of protection against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for the coronavirus disease 2019 (COVID-19). However, it is unknown whether protection is contributed by antibody effector functions alone.
 Study: An anti-SARS-CoV-2 non-neutralizing antibody with Fc-effector function defines a new NTD epitope and delays neuroinvasion and death in K18-hACE2 mice. Image Credit: ktsdesign / Shutterstock.com
Study: An anti-SARS-CoV-2 non-neutralizing antibody with Fc-effector function defines a new NTD epitope and delays neuroinvasion and death in K18-hACE2 mice. Image Credit: ktsdesign / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
In a recent study published on the preprint server bioRxiv*, researchers isolated a non-neutralizing antibody (CV3-13) from a patient who had recovered from COVID-19 and possessed potent Fc-mediated effector functions which target the N-terminal domain (NTD) of the SARS-CoV-2 spike (S) protein. Cryo-electron microscopic (cryo-EM) images revealed that when the CV3-13 antibody is in a complex with the SARS-CoV-2 S protein, a specific angle of approach was taken by the antibody to bind to a novel NTD epitope. This binding caused an overlap to occur with an NTD supersite that is a location of frequent mutations in SARS-CoV-2.
These researchers subsequently utilized genetically modified mouse models to demonstrate the ability of CV3-13 to delay the invasion of the nervous system and death from SARS-CoV-2 in a prophylactic setting. Notably, CV3-13 was not found to alter the replication dynamics of SARS-CoV-2.
CV3-13 binds to the NTD of SARS-CoV-2 S glycoprotein
In the current study, the authors isolated non-neutralizing antibodies from a patient who had recently recovered from COVID-19 to test if these antibodies could provide protection against SARS-CoV-2 alone. From the donor patients’ peripheral blood mononuclear cells, the authors used fluorescent SARS-CoV-2 S 2P as a probe to sort 432 antigen-specific B-cells, from which 27 monoclonal antibodies were generated.
The CV3-13 antibody was shown to bind to the S protein; however, it failed to neutralize the SARS-CoV-2 pseudovirus. The authors subsequently exposed different S protein variants on the surface of transfected cells to determine if CV3-13 would bind to them and which epitope the antibody recognizes. The D514G and wild-type variants were both efficiently bound by CV3-13; however, the S protein of the Alpha variant was unrecognized by the antibody.
The epitope of CV3-13 was determined by the authors using this differential binding to their advantage by initiating B.1.1.7 variant mutations into the wildtype variant S protein. The CV3-13 antibody was shown to recognize all mutations, apart from the Δ144 mutant caused by a single amino acid deletion in the S1 NTD.
Is CV3-13 a non-neutralizing antibody?
The capability of CV3-13 to neutralize a pseudovirus carrying the SARS-CoV-2 S protein was tested to confirm if CV3-13 was a non-neutralizing antibody. The authors’ analysis showed that CV3-13 was unable to neutralize the pseudo viral particles.
The author induced L234A/L235A (LALA) and G236A/S239D/A330L/I332E (GASDALIE) mutations to the Fc portion of CV3-13; however, this had no effect on the ability of the antibody to recognize the S proteins or its modified profile. In fact, the GASDALIE mutations were found to strengthen the interaction between the FcγRs and IgG Fc portion, whereas the LALA mutations weaken them.
The authors then tested the ability of CV3-13 to possibly mediate Fc-effector functions by using an antibody-dependent cellular cytotoxicity assay. This assay involves the use of a human T-lymphoid cell line that is resistant to natural killer cell-mediated lysis and stably expressing the full-length S protein on their surface target cells.
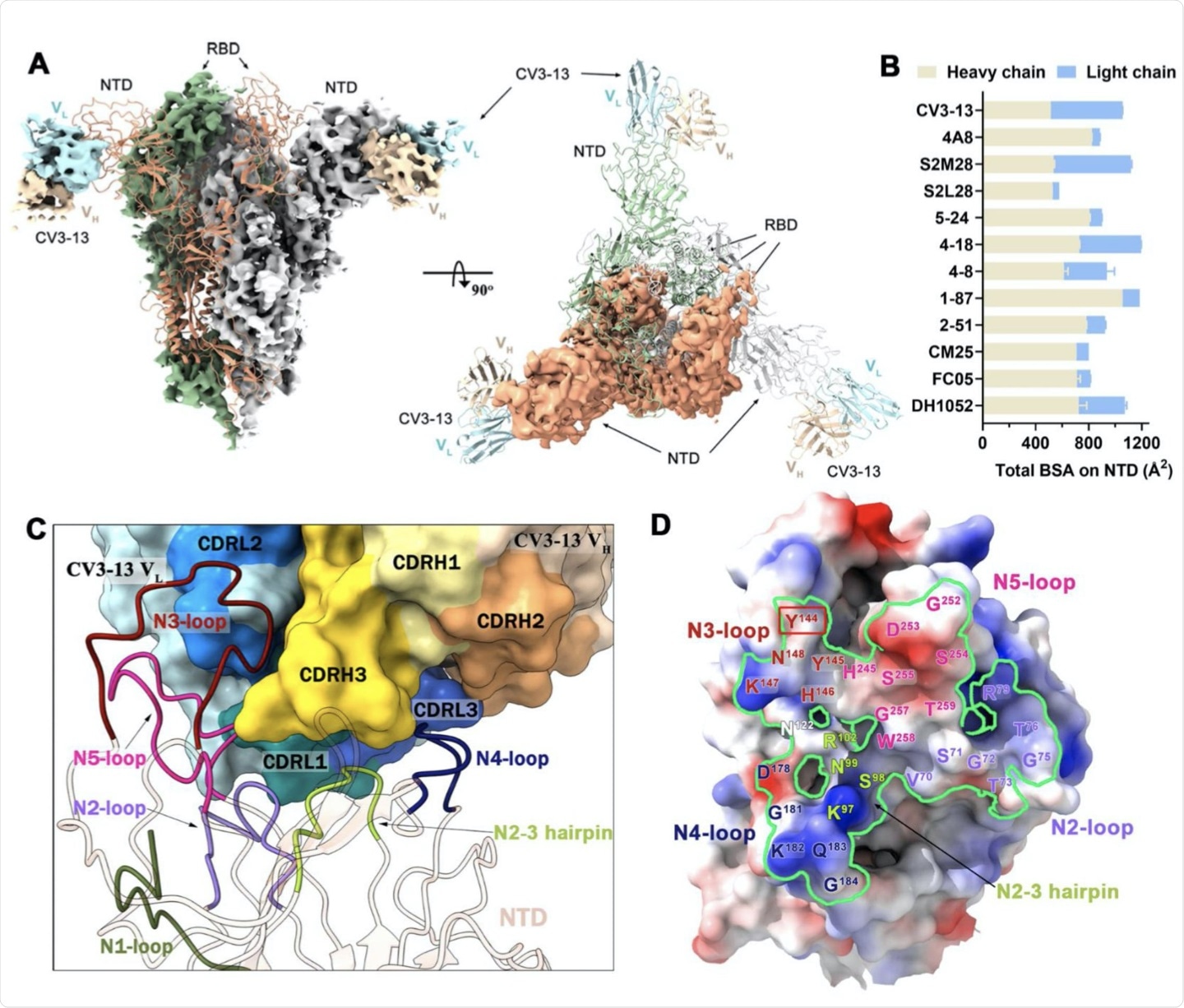 CV3-13 distinct angle of approach to novel NTD epitope
CV3-13 distinct angle of approach to novel NTD epitope
The authors attempted to determine how the distinct angle of approach of CV3-13 and the antibody recognition site affects the mode of action by aligning the NTD-Fv portions of CV3-13 with other known NTD-directed antibodies. The angle in which the CV3-13 antibody approaches the NTD is almost perpendicular relative to the S trimer axis, with its epitope footprint overlapping the NTD supersite.
The CV3-13 antibody accesses the NTD via the top of the S trimer and the antibodies that target the infectivity enhancing site access the NTD via the bottom of the S protein, which is closer to the viral membrane. When the angles of approach are calculated, the contrast in binding mechanisms of CV3-13 and other NTD-specific antibodies is clear. The supersite binding neutralizing antibodies approach the NTD with an angle in the range of 6° - 15°, which is significantly different than the angle of approach by CV3-13, which is around 30°.
The angle that CV3-13 utilizes situates this antibody in a position between the binding angle of the antibodies that recognize the neutralization supersite, which binds towards the top of the S protein. The antibodies are then able to target the infectivity enhancing site, which binds towards the bottom end of the S protein in closer vicinity to the viral membrane.
Summary
This study revealed that the CV3-13 antibody can mediate Fc effector functions against S protein-expressing cells; however, it was unable to neutralize pseudo viral particles. It is suggested by the authors that the approaching angle and fine epitope specificity utilized by CV3-13, as compared to neutralizing NTD-specific monoclonal antibodies, limits its capacity to sterically hinder Spike-co-receptor/auxiliary receptor interactions. A suggested model of neutralization for other NTD binding antibodies is the prefusion-to-postfusion transition of the S protein.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Beaudoin-Bussieres, G., Chen, Y., Ullah, I., et al. (2021). An anti-SARS-CoV-2 non-neutralizing antibody with Fc-effector function defines a new NTD epitope and delays neuroinvasion and death in K18-hACE2 mice. bioRxiv. doi:10.1101/2021.09.08.459408. https://www.biorxiv.org/content/10.1101/2021.09.08.459408v1
- Peer reviewed and published scientific report.
Beaudoin-Bussières, Guillaume, Yaozong Chen, Irfan Ullah, Jérémie Prévost, William D. Tolbert, Kelly Symmes, Shilei Ding, et al. 2022. “A Fc-Enhanced NTD-Binding Non-Neutralizing Antibody Delays Virus Spread and Synergizes with a NAb to Protect Mice from Lethal SARS-CoV-2 Infection.” Cell Reports 38 (7). https://doi.org/10.1016/j.celrep.2022.110368. https://www.cell.com/cell-reports/fulltext/S2211-1247(22)00089-4.