The severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is a beta-coronavirus whose emergence has led to the coronavirus 19 (COVD-19) global pandemic. As of September 15, 2021, over 227 million people have been infected with SARS-CoV-2, worldwide with almost 4.7 million casualties.
Additionally, the emergence of the new SARS-CoV-2 variants can infect vaccinated individuals, as well as cause re-infection in individuals who have previously recovered from COVID-19.
The symptoms associated with COVID-19 range from mild to severe to life-threatening. Mild forms of COVID-19 may be either asymptomatic or caused mild symptoms that are often associated with the common cold.
Comparatively, the severe forms of COVID-19 can lead to symptoms ranging from fever, fatigue, and malaise to gastrointestinal, pulmonary, dermatological, and neurological disorders. COVID-19 is considered to be life-threatening when there is an incidence of pneumonia. Pneumonia can lead to acute respiratory distress syndrome (ARDS), which may require mechanical ventilation and oxygen support for survival.
Pathophysiological and immune profiling of hosts has indicated that a dysregulated immune response leads to the life-threatening cytokine storm and subsequent immune paralysis, both of which can increase the patient’s risk of multiple organ failure and death. Although mild and moderate cases of COVID-19 do not cause excessive immune activation, recent data suggests that COVID-19 symptoms can remain in about 30% of patients who have recovered from mild COVID-19 infection.
Although several studies have been conducted on the differences in pathological profiles of severe and mild-to-moderate COVID-19, the determination of longitudinal trajectories involving the circulating immune landscape is still not fully understood.
A new study published on the preprint server medRxiv* includes an analysis of plasma cytokine levels, peripheral blood immune cell composition, and SARS-CoV-2 specific immunoglobulins (Igs) in mild-to-moderate cases of COVID-19. Distinct disease phases, along with their immunological and serological layout, were also characterized in this study.
About the study
The current study included COVID-19 patients who were recruited either from the local testing center or from the division of tropical medicine and infectious diseases. Peripheral blood was obtained from these patients on the day of testing or hospitalization, as well as on day two and day six from the day of testing or hospitalization. All individuals who tested negative for COVID-19 were considered as healthy controls and provided blood only on the day of testing.
Blood samples were then analyzed by flow cytometry and multi-pathway phosphoprotein analyses. Plasma samples were also obtained from the collected blood using centrifugation, which was then used to determine the presence of SARS-CoV-2 specific antibodies. Finally, cytokine concentrations were also determined from the plasma samples.
Study findings
The analysis of longitudinal trajectories led to the determination of three different stages in patients who experienced mild-to-moderate courses. The first stage is the incubation period, during which manifestation of the symptoms took place that led to testing or hospitalization in case of elderly patients.
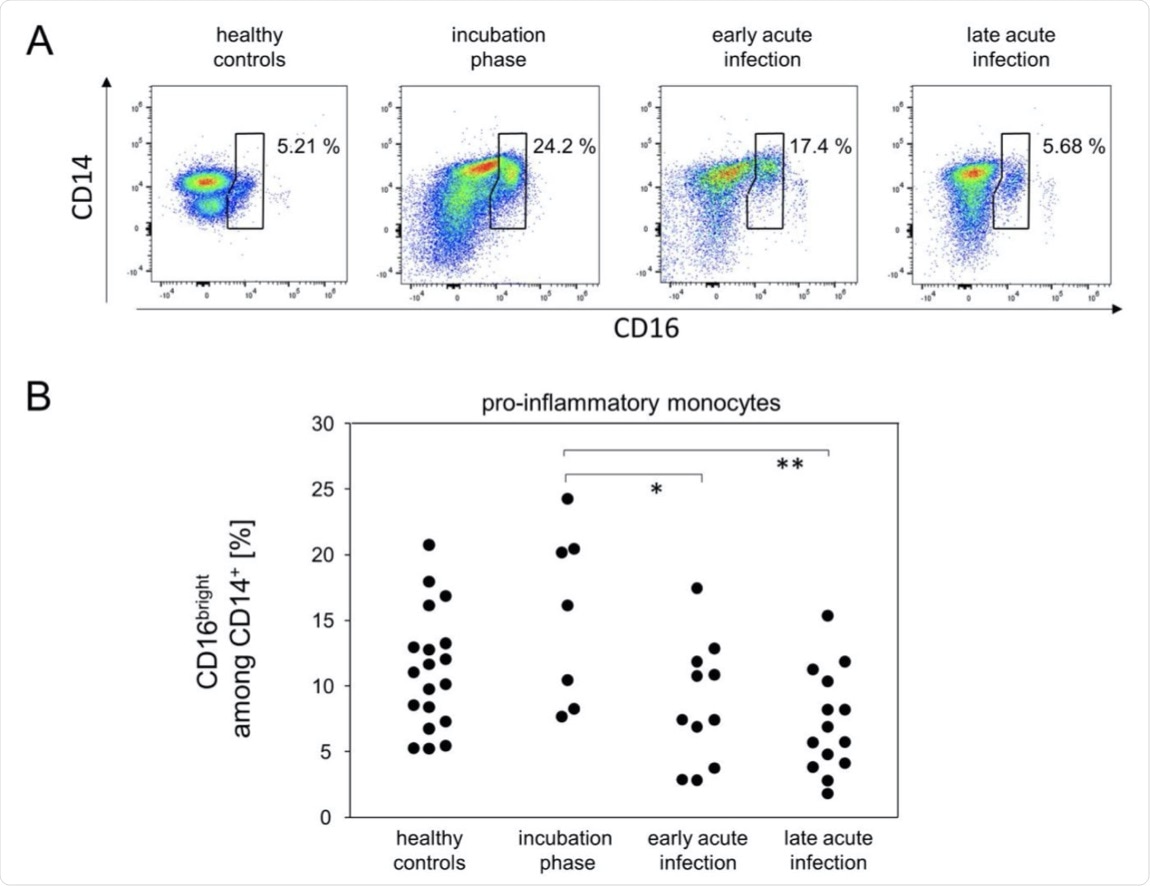 Pro-inflammatory monocytes were more abundant during the incubation phase. (A) Representative pseudocolor plots of CD14 and CD16 expression data of SSCmed monocytes demonstrated kinetics for the enrichment of pro-inflammatory monocytes (polygonal gate). (B) Quantitative data of pro-inflammatory monocyte proportions. *p < 0.05; **p < 0.01; Tukey-Kramer test.
Pro-inflammatory monocytes were more abundant during the incubation phase. (A) Representative pseudocolor plots of CD14 and CD16 expression data of SSCmed monocytes demonstrated kinetics for the enrichment of pro-inflammatory monocytes (polygonal gate). (B) Quantitative data of pro-inflammatory monocyte proportions. *p < 0.05; **p < 0.01; Tukey-Kramer test.
The incubation stage was followed by the early acute infection stage, which was characterized by high levels of peripheral plasmablasts (CD27+ and CD38bright) and exhausted cytotoxic T-cells. During this stage, virus-specific antibodies were also detected.
The immune response during this initial phase was characterized by an increase in cytotoxic T-cells (CD38+), pro-inflammatory monocytes (CD16bright and CD14+), and serum IP-10, along with a decrease in the number of peripheral T-helper cells.
The final stage was the late acute infection stage, during which most of the aforementioned immune cells return to their normal levels, except the virus-specific antibodies. The levels of immature neutrophils was also found to rise during the late acute infection stage.
The results of the current study also indicated that while the level of peripheral plasmablasts increased between day 1 and 9 of the onset of symptoms, detection of SARS-CoV-2 specific antibodies took place between 3 to 6 days of the onset of symptoms. Among the antibodies, IgM levels were found to reach a plateau during the early acute infection stage, whereas IgG and IgA levels were still found to rise during the late acute infection stage.
The study did not find any major changes among the T helper cell subpopulations. However, significant changes were observed among the cytotoxic T-cell populations.
More specifically, the researchers observed an increased expression of CD38, along with CD27- cytotoxic T-cells, which subsequently declined during the later stages.
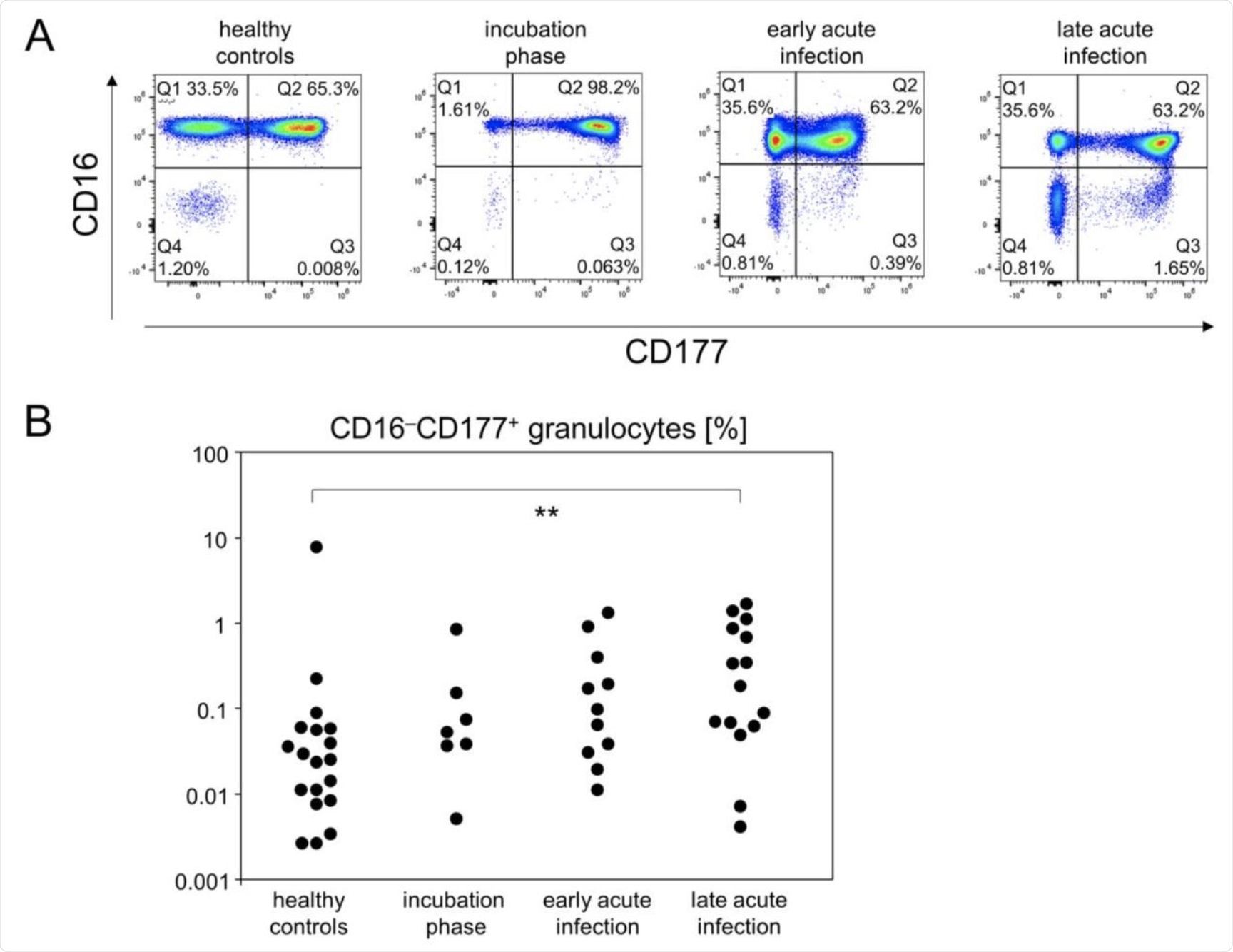 The portion of CD16−CD177+ granulocytes gradually increased until the late acute infection phase. (A) Representative pseudocolor plots of CD16 and CD177 expression data of SSChi leukocytes showed that CD16−CD177+ granulocytes specifically emerged at the late acute infection phase (Q3 gate). (B) Quantitative flow cytometry data indicated gradually increasing abundances of CD16−CD177+ granulocytes. **p < 0.01; Tukey-Kramer test.
The portion of CD16−CD177+ granulocytes gradually increased until the late acute infection phase. (A) Representative pseudocolor plots of CD16 and CD177 expression data of SSChi leukocytes showed that CD16−CD177+ granulocytes specifically emerged at the late acute infection phase (Q3 gate). (B) Quantitative flow cytometry data indicated gradually increasing abundances of CD16−CD177+ granulocytes. **p < 0.01; Tukey-Kramer test.
Additionally, increased expression of PD-1+ cytotoxic T-cells was observed during early acute infection. The presence of these cells may be responsible for the impairment of T-cell function. Increased differentiation into effector memory and stem cell memory was also observed.
Furthermore, it was observed that a decrease in the level of peripheral T-cells did not correspond to an increase in the number of apoptotic cells. Taken together, these findings suggest that the T-cells were not eliminated, and were instead relocated to sites of infection. No alteration in natural killer (NK) cells, monocyte/granulocyte, or monocyte/NK was observed.
“Upcoming research needs to focus on comparable disease stages during severe COVID-19 in order to allow for the identification of landmarks where resolution of viral infection segregates from progressing immune dysregulation and fatal outcomes.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Müller, M., Volzke, J., Subin, B., et al. (2021). Distinguishing Incubation and Acute Disease Stages of Mild-to-Moderate COVID-19. medRxiv. doi:10.1101/2021.09.07.21263200. https://www.medrxiv.org/content/10.1101/2021.09.07.21263200v1
- Peer reviewed and published scientific report.
Müller, Michael, Johann Volzke, Behnam Subin, Christian Johann Schmidt, Hilte Geerdes-Fenge, Emil Christian Reisinger, and Brigitte Müller-Hilke. 2022. “Distinguishing Incubation and Acute Disease Stages of Mild-To-Moderate COVID-19.” Viruses 14 (2): 203. https://doi.org/10.3390/v14020203. https://www.mdpi.com/1999-4915/14/2/203.