The ongoing coronavirus disease 2019 (COVID-19) pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus has a high transmission rate, and its symptoms may vary from being mild to severe pneumonia-like. Some people are also asymptomatically infected with the virus. Common clinical manifestations of mild COVID-19 include cough, fever, muscular pain, loss of smell and taste, shortness of breath, vomiting, and sore throat. Severe infection may lead to organ failure, respiratory failure, and eventually death. Scientists have indicated that the virus alone does not cause mortality. The cytokine storm is responsible for respiratory failure, multiple organ failure, or heart attack that leads to death.
 Study: Neurokinin-1 Receptor as a potential drug target for COVID-19 treatment. Image Credit: sdecoret/ Shutterstock
Study: Neurokinin-1 Receptor as a potential drug target for COVID-19 treatment. Image Credit: sdecoret/ Shutterstock
COVID-19 treatments using Neurokinin-1 Receptor antagonist as a drug
Scientists worldwide have been developing effective COVID-19 treatments. Several clinical trials are ongoing to evaluate various drugs with antiviral and anti-inflammatory effects. A new opinion article proposes that Substance P (SP), via Neurokinin-1 Receptor (NK-1R), is responsible for the cytokine storm and inflammation in the case of severe COVID-19 infection.
An opinion article published in Biomedicine & Pharmacotherapy aims to provide evidence that SP could be the possible factor for initiating the cytokine storm during severe SARS-CoV-2 infection. They have also proposed that such a condition could be effectively treated using Neurokinin-1 Receptor antagonist, aprepitant, as a drug.
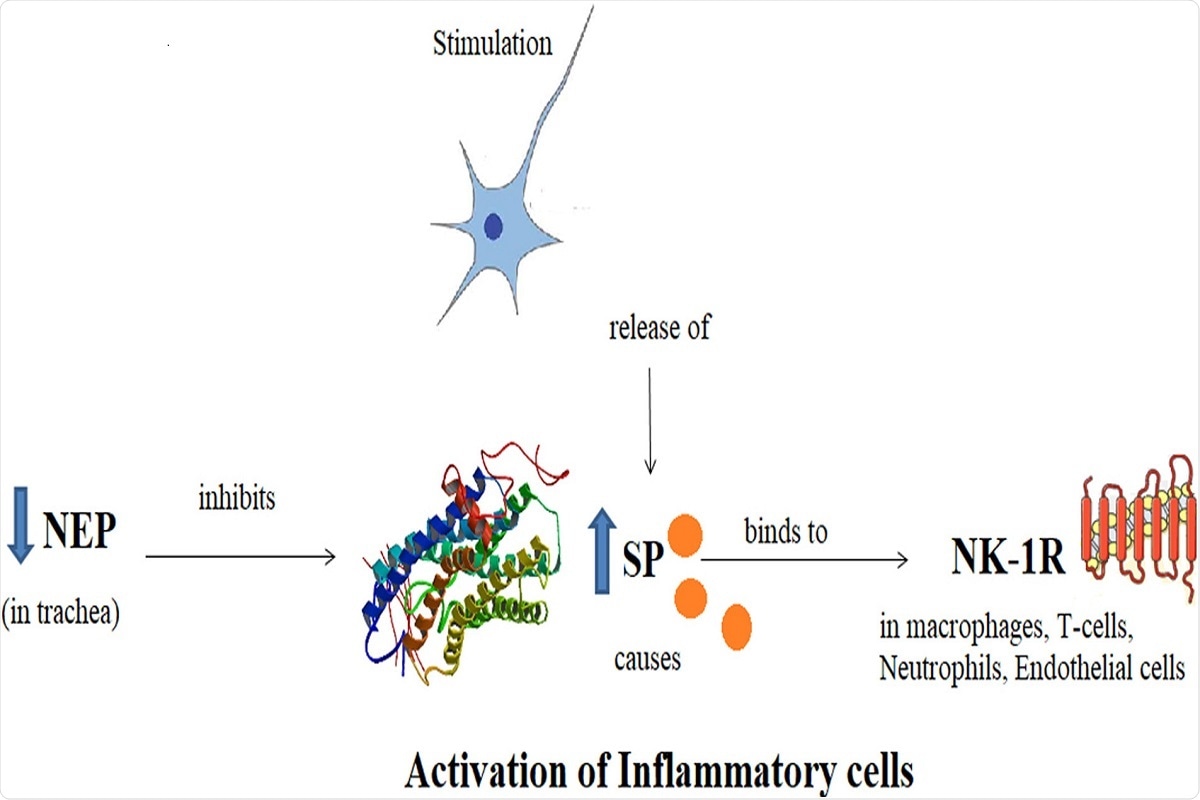 Graphical Abstract: Schematic diagram showing Substance P, its receptor Neurokinin-1 and Neutral Endopeptidase. SP binds to NK-1R as a result of nociceptive stimulus and potentiates the inflammation. NEP acts as a regulatory mechanism by degrading the SP and hence, inflammation.
Graphical Abstract: Schematic diagram showing Substance P, its receptor Neurokinin-1 and Neutral Endopeptidase. SP binds to NK-1R as a result of nociceptive stimulus and potentiates the inflammation. NEP acts as a regulatory mechanism by degrading the SP and hence, inflammation.
The link between Substance P and Neurokinin-1 Receptor
Euler and Gaddum first discovered SP as a brain-gut hormone in 1931. Extensive research revealed different roles of SP as a neuromodulator, neurotransmitter, and neurohormone, encoded by the gene named Tachykinin-1 (TAC-1). Additionally, SP has many other functions, such as autocrine, paracrine, and endocrine functions. Scientists reported that SP and NK-1R are highly expressed in the central and peripheral nervous systems and the cardiovascular system. Immune cells such as leukocytes, lymphocytes, monocytes, and macrophages can also express SP. SP is a chemokine that stimulates the cytokine release in respiratory tracts after attaching with NK-1R. These are also associated with various pathological conditions and inflammation.
Scientists revealed that NK-1R is a 7-transmembrane domain, G-protein coupled receptor that possesses a high SP affinity. This receptor is also present in many cells such as white blood cells, lymphatics neurons, fibroblasts, vascular endothelium and, cardio-ventilatory regulatory centers. Previous research revealed that after the formation of the SP/NK1-R complex, a signaling cascade is responsible for the production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG).
Possible role of SP/Neurokinin 1-Receptor in COVID-19 infection
Some of the early symptoms associated with COVID-19, such as cough, loss of smell and taste, headache, sore throat, are linked with the release of SP from trigeminal ganglion via TrN. Also, the release of the SP in the vagal C fibers in the larynx and upper respiratory tracts causes a cough. Scientists have found that NK-1R antagonists could reduce the refractory cough frequency.
Differential severity of SARS-CoV-2 infection in varied age groups has also been linked with the expression of SP. Mostly, SARS-CoV-2 is found to severely affect older age groups, compared to younger people. Many studies have indicated that the elderly tend to have elevated SP levels and that a higher concentration of SP leads to death.
Scientists have revealed that individuals with co-morbidities such as diabetes mellitus (DM), hypertension (HTN), hepatic renal and cardiovascular conditions are more vulnerable to severe COVID-19 infection. Generally, patients with DM and HTN are treated with Dipeptidyl peptidase-4 (DPP4) and Angiotensin-converting enzyme (ACE) inhibitors. The spike proteins of the virus attach to the ACE2 of the host to establish infection.
Therefore, ACE2 is the main receptor associated with SARS-CoV-2 infection, which is abundantly present in the lung capillaries, renal and endothelial cells of humans. A high level of SP dilates vessels, resulting in more blood pumping in the heart, thereby increasing the risks of a heart attack. Increased levels of SP also lower the blood sugar level, causing multiple organ failure in severe COVID-19 cases. Also, previous research revealed that SP secretion by the immune cells is positively correlated with the viral load.
Earlier studies had indicated SP enhances inflammation via various mechanisms such as vasodilation and increased vascular permeability, leukocyte extravasation, and immune response activation in native cells and pathogens. In mice models, NK-1R deficient mice showed pulmonary inflammation as compared to controls.
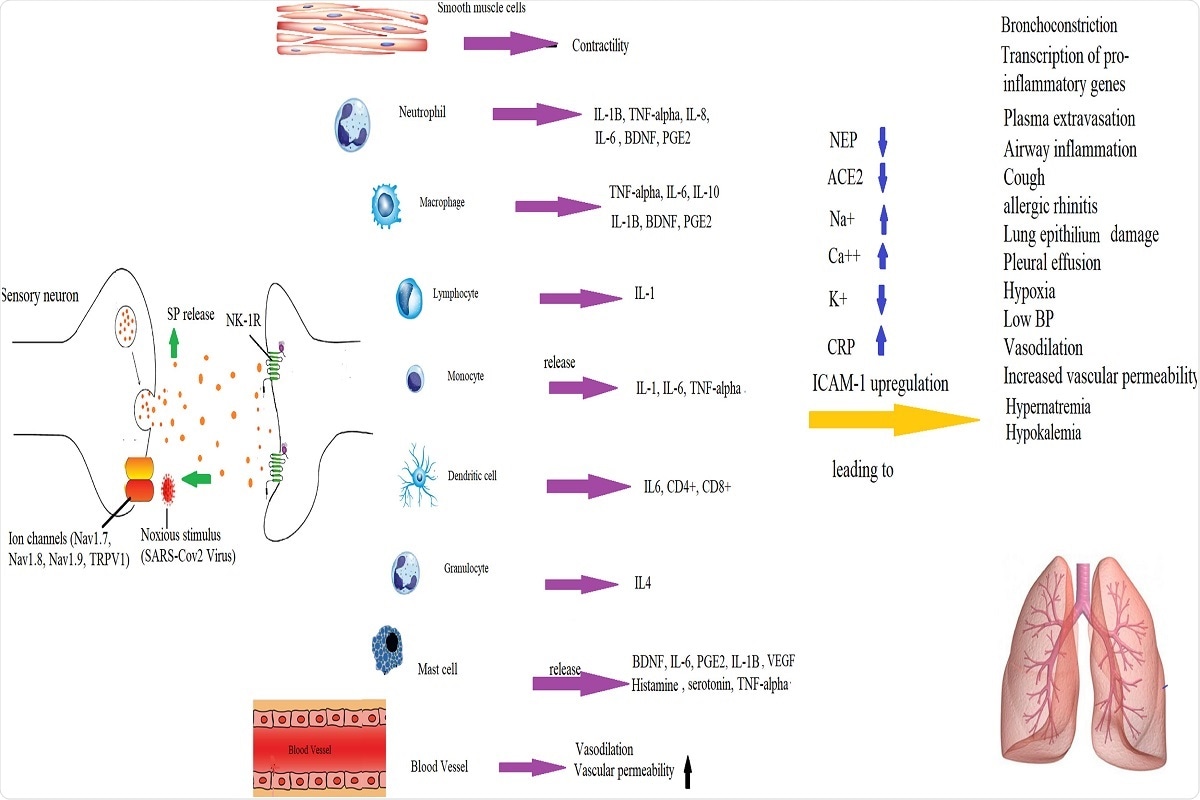 Fig. 3. Mechanisms by which SP-induced inflammation is implicated in the pathogenesis of COVID-19 infection. SP binds to NK-1R on endothelial cells to increase BBB permeability and release of cytokines by immune cells.
Fig. 3. Mechanisms by which SP-induced inflammation is implicated in the pathogenesis of COVID-19 infection. SP binds to NK-1R on endothelial cells to increase BBB permeability and release of cytokines by immune cells.
Management of COVID-19 infection
The author of this study strongly suggested that SP/NK-1R complex is associated with the pathogenesis of COVID-19 infection. A recent randomized clinical trial associated with the evaluation of NK-1R antagonists (Dexamethasone and Aprepitant) for treating COVID-19 infection revealed that both the drugs showed improved clinical outcomes. Also, patients who received the combination of these drugs reported improved clinical symptoms and reduced C-reactive protein.
Journal reference:
- Mehboob, R. (2021) "Neurokinin-1 Receptor as a potential drug target for COVID-19 treatment", Biomedicine & Pharmacotherapy, 143, p. 112159. doi: 10.1016/j.biopha.2021.112159.