Even with the rapid production and distribution of safe and effective coronavirus disease 2019 (COVID-19) vaccines, their availability in low-resource settings is an issue due to production, affordability, and allocation challenges. Therefore, it is important to identify inexpensive, effective, and widely available COVID-19 therapies.
 Study: Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Image Credit: mdbildes/ Shutterstock
Study: Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Image Credit: mdbildes/ Shutterstock
The drug fluvoxamine, which is a selective serotonin reuptake inhibitor (SSRI), is a possible treatment option against COVID-19 because of its anti-inflammatory and possible antiviral effects. A previous placebo-controlled, randomized trial highlighted that fluvoxamine could potentially reduce the risk of clinical deterioration in outpatients with COVID-19.
In a study published in the Lancet, a team of researchers from Brazil conducted a randomized, placebo-controlled, adaptive platform trial to evaluate the efficacy of fluvoxamine to prevent COVID-19 progression and hospitalization. The flexible platform trial implemented by the authors permits the addition of additional agents and testing with standardized operating procedures outlined in a single overarching master protocol.
The study
To date, there have been nine thousand eight hundred and three potential participants screened for inclusion in this trial. There were one thousand four hundred and ninety-seven participants enrolled by August 5, 2021. Seven hundred and forty-one received fluvoxamine, and seven hundred and fifty-six received the placebo.
Seventy-nine (11%) participants in the fluvoxamine group had primary outcome events compared to one hundred and nineteen (16%) in the placebo group. The majority of these events were hospitalizations. Evidence gained via Bayesian beta-binomial model suggested there are benefits associated with the use of fluvoxamine for reducing the composite primary endpoint of hospitalization, which was defined as retention in a COVID-19 emergency setting to transfer to a tertiary hospital due to COVID-19 related issues.
For the intention to treat (ITT) population, the probability that the event rate was lower in the fluvoxamine group compared with the placebo group was 99.8% and 99.7 for the modified ITT (mITT). It was recommended by the independent data safety monitoring committee (DSMC) on August 5, 2021, that the authors should stop randomly assigning participants to the fluvoxamine group because this comparison had met the prespecified superiority criterion for the primary endpoint.
Tolerability issues caused eighty-four fluvoxamine participants and sixty-four placebo participants to stop involvement in the trial. It was shown in the per-protocol findings that participants who displayed optimal adherence indicated significant treatment effects regarding the primary outcome and mortality.
There were no significant differences in the occurrence of treatment emergent adverse effects between participants in the fluvoxamine and placebo groups. Within the subgroups (age, sex, days since the onset of symptoms, smoking status, and comorbidities), there is no evidence of moderation of treatment effect for fluvoxamine compared with the placebo.
The results from this study were consistent with an earlier trial conducted on smaller sample size. The previous study also used fluvoxamine but at a higher dose and included a group of lower-risk individuals for the primary outcome and found no clinical deterioration among eighty participants who received fluvoxamine vs. six cases among seventy-two patients who received a placebo. Another previous study conducted in France showed how treating COVID-19 patients with SSRIs reduced intubation and death.
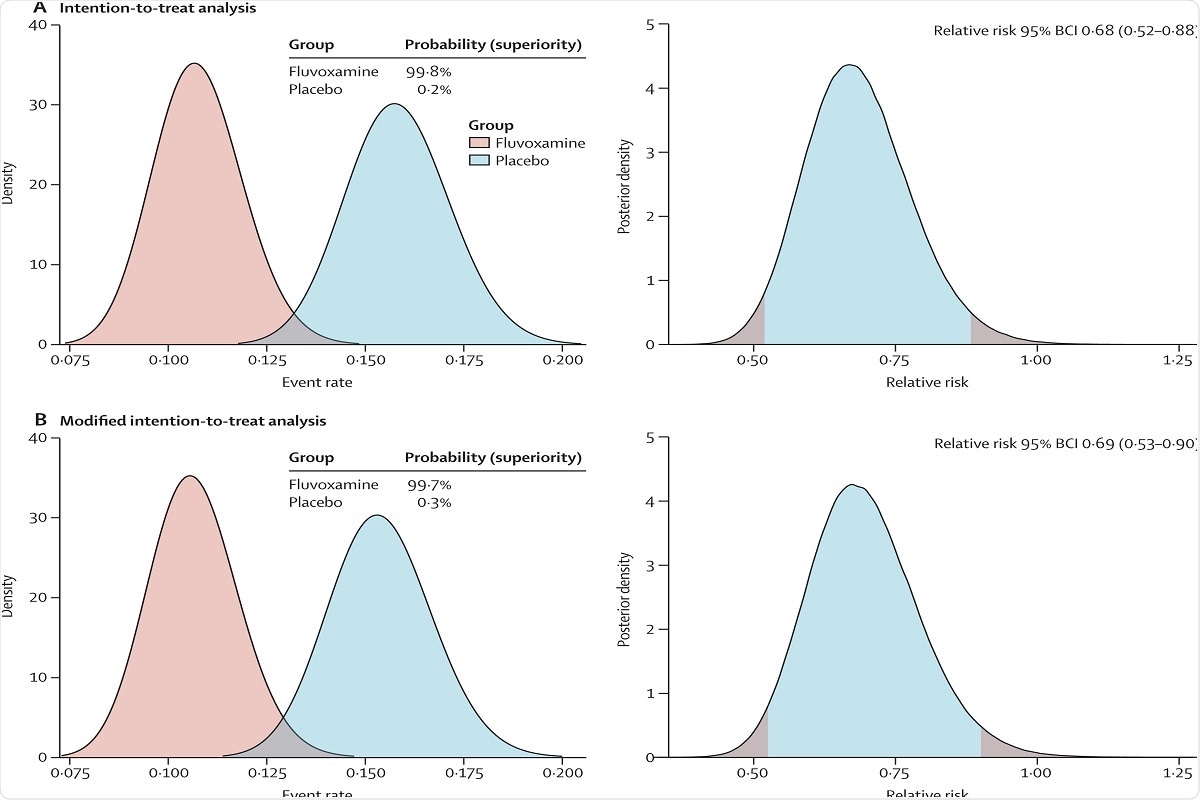 Figure 2: Probability of efficacy and Bayesian relative risk of hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to tertiary hospital due to COVID-19 for fluvoxamine versus placebo.
Figure 2: Probability of efficacy and Bayesian relative risk of hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to tertiary hospital due to COVID-19 for fluvoxamine versus placebo.
Implications
The use of therapeutic interventions such as fluvoxamine to limit the progression of diseases and possibly prevent hospitalization is critically dependent on identifying higher-risk individuals. There is a lower risk associated with unselected populations. It remains unclear what absolute reduction in risk of clinical deterioration would motivate people to choose such treatment methods. These considerations highlight the importance of developing a validated prediction rule for deterioration in patients in the early stages of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.