Whenever a pathogen invades a host, the adaptive immune system elicits an immune response tailored against that pathogen. This specificity arises because of T-cells and B-cells of the immune system. Since this response is cell-based, adaptive immunity provides immunological memory.
Even though adaptive immunity is pathogen-specific, there is a possibility of cross-reactivity, in which the immune response against one pathogen can recognize another pathogen. This cross-reactivity, which is otherwise known as heterologous immunity, arises due to the presence of related antigenic epitopes shared by different pathogens and the cross-reactivity of antigen receptors.
 Study: Heterologous humoral immunity to human and zoonotic coronaviruses: Aiming for the achilles heel. Image Credit: Lightspring / Shutterstock.com
Study: Heterologous humoral immunity to human and zoonotic coronaviruses: Aiming for the achilles heel. Image Credit: Lightspring / Shutterstock.com
In a recent article published in Seminars in Immunology, the authors review the heterologous immunity to coronaviruses and the extent of immune cross-reactivity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Human coronaviruses
Due to the devastating effects of the coronavirus disease 2019 (COVID-19) pandemic, there is an urgent need to understand coronavirus infection and host immunity. This will also help in understanding the pathogenic potential of SARS-CoV-2.
Seven coronaviruses have made humans their host, including HCoV-OC43 and HCoV-229E, which were isolated in 1965, SARS-CoV, which was discovered in 2002, HCoV-NL63 and HCoV-HKU1 that were discovered in 2004-2005, and the Middle East respiratory syndrome coronavirus (MERS-CoV) that emerged in 2012 and was pathogenic with limited transmissibility. SARS-CoV-2, which was first identified in 2019, has had the greatest impact by far of all human coronaviruses (HCoVs). Notably, SARS-CoV, MERS-CoV, and SARS-CoV-2 were all of zoonotic or animal origin.
All coronaviruses enter into the host cell via the interaction of the viral spike protein (S) with the host receptor. The host receptors vary for the different coronaviruses
Humans are frequently exposed to animal coronaviruses through bats, avian, canine, and feline souces. Exposure to a coronavirus may have the potential to induce adaptive immune responses that can cross-react with other coronaviruses and SARS-CoV-2. In fact, one study has demonstrated that individuals with no history of SARS-CoV or SARS-CoV-2 infection have SARS-CoV-2-reactive T-cell responses, which may be due to exposure to unknown animal coronaviruses.
Coronavirus cross-reactive antibodies
Antibody targets
Serology assays are used to detect coronavirus infections. Due to these assays, antibody cross-reactivity between HCoVs and zoonotic coronaviruses was identified. The host immune system predominantly generates antibodies against the S protein and nucleoproteins (N) of coronaviruses.
Both S and N proteins have been implicated in antibody cross-reactivity between SARS-CoV-2 and HCoVs. S has two subunits including the S1 subunit, which contains the receptor-binding domain (RBD), and the S2 subunit, which is highly conserved among HCoVs and animal coronaviruses.
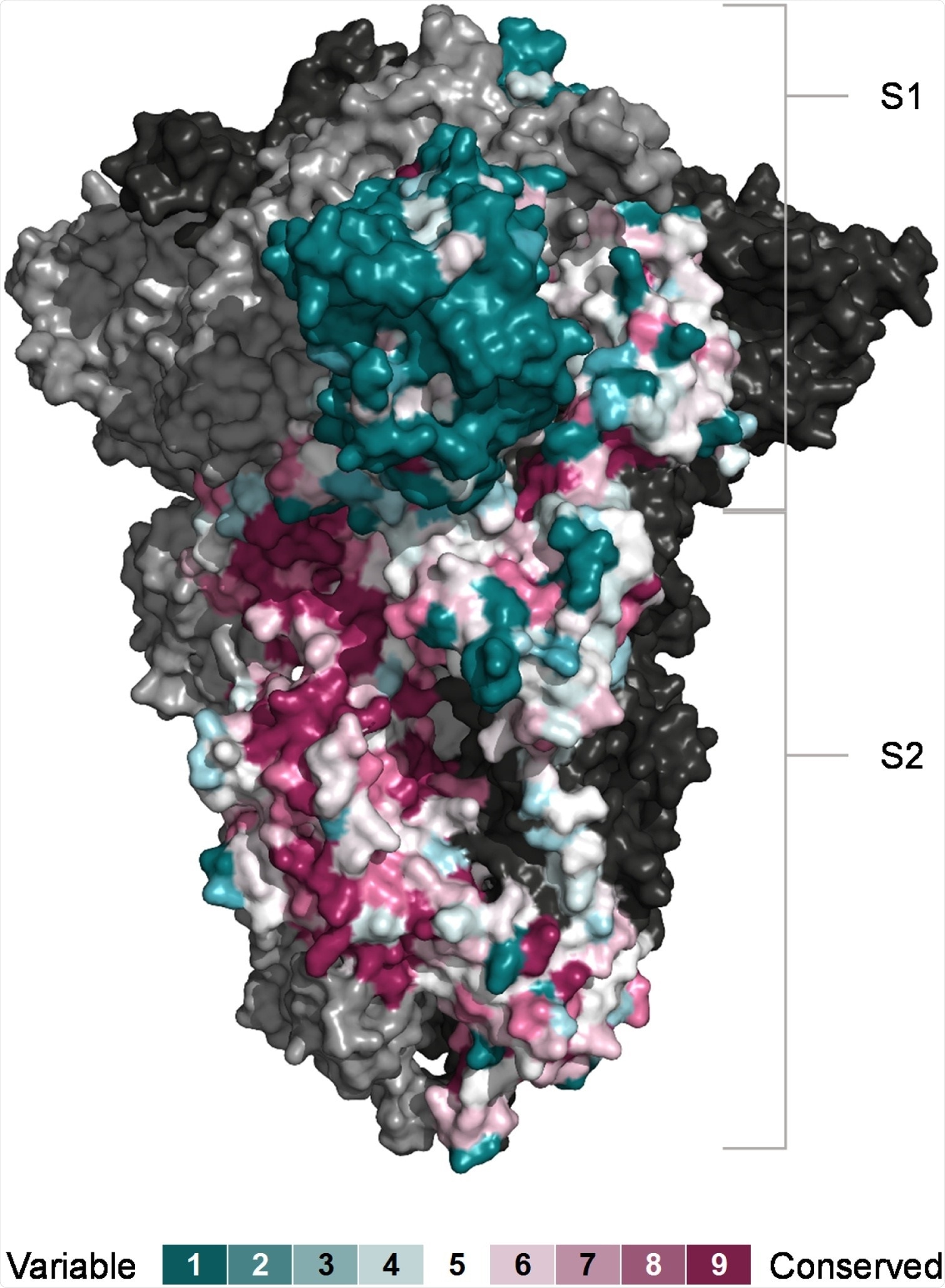 Conservation of SARS-CoV-2 S subunits. Sequence conservation visualised on the closed structure of SARS-CoV-2 spike (PDB ID 6ZGE) [119]. Chain A was coloured according conservation calculated with the ConSurf algorithm (https://consurf.tau.ac.il) from the sequences of 24 animal and human coronaviruses. The other two chains are depicted in ling and dark grey respectively.
Conservation of SARS-CoV-2 S subunits. Sequence conservation visualised on the closed structure of SARS-CoV-2 spike (PDB ID 6ZGE) [119]. Chain A was coloured according conservation calculated with the ConSurf algorithm (https://consurf.tau.ac.il) from the sequences of 24 animal and human coronaviruses. The other two chains are depicted in ling and dark grey respectively.
Pre-existing SARS-CoV-2 S1 and RBD cross-reactive antibodies
SARS-CoV-2 RBD and N cross-reactive antibodies have been observed in 0.9 % and 16% of pre-pandemic patient samples. A similar study in a different group of patients reported SARS-CoV-2 RBD and N cross-reactive antibodies in 0.6% and 24% of samples. Another study detected SARS-CoV-2 RBD and N cross-reactive antibodies in 44% and 100% of pre-pandemic samples.
More than 80% of antibodies generated are against the SARS-CoV-2 S2 and N-terminal domain (NTD), whereas anti-RBD antibodies are a small minority.
Even though pre-existing cross-reactive antibodies against SARS-CoV-2 S1 or RBD epitopes have been detected in samples, their levels and frequency are generally lower than that of pre-existing antibodies to SARS-CoV-2 S2 or other SARS-CoV-2 antigens. Therefore, these antibodies are not extensively studied.
Pre-existing SARS-CoV-2 S2 cross-reactive antibodies
SARS-CoV-2 S cross-reactive antibodies have been observed in a proportion of pre-pandemic serum samples. Pre-pandemic serum samples contained lower levels of SARS-CoV-2 S-binding antibodies that were predominantly against the S2 subunit.
Conversely, COVID-19 convalescent serum samples contained SARS-CoV-2 S-binding antibodies targeted against the S2 and S1 subunits. Notably, SARS-CoV-2 S-binding antibodies were detected in around 5% of adults and about 44% of children and adolescents.
SARS-CoV-2 N-binding antibodies were also detected in a comparable proportion of pre-pandemic serum samples. However, the same serum samples did not have SARS-CoV-2 S-binding antibodies, thereby suggesting that the two are not always linked.
Several studies have reported the detection of cross-reactive antibodies against various portions of SARS-CoV-2 S2 in pre-pandemic serum samples. The percentage of samples with these cross-reactive antibodies varies based on the S2 region or domain, mode of infection (natural versus immunization), age of patients, method of detection, geographic or regional patterns of HCoV spread, and the time of sample collection.
In conclusion, the presence of antibody cross-reactivity with SARS-CoV-2 S and S2 point to previous exposure to HCoVs.
Induction of HCoV cross-reactive antibodies by SARS-CoV-2 infection or vaccination
Antibodies produced from either SARS-CoV-2 infection or vaccination can induce that cross-reactivity with other coronaviruses, a phenomenon the authors of the current study refer to as ‘back-boosting’ Several studies during the COVID-19 pandemic have reported the back-boosting effect and identified specific targets of cross-reactive antibodies.
There is a bidirectional nature to the cross-reactive antibody induction between HCoVs and SARS-CoV-2. The strong back-boosting of HCoV cross-reactive antibodies following SARS-CoV-2 infection or vaccination greatly facilitates their detection.
Pre-existing SARS-CoV-2 S cross-reactive B cells
Antibodies are produced by B-cells. Heterologous immunological memory is also demonstrated by the detection of B-cells.
Similar to the antibody studies, B-cells against SARS-CoV-2 and other HCoVs were detected in pre-pandemic samples. Likewise, a SARS convalescent donor showed the presence of B-cells that displayed SARS-CoV-2 and HCoV reactivity.
SARS-CoV-2 RBD-reactive antibodies may exist prior to exposure to SARS-CoV-2; however, antibodies that cross-reactive with more conserved regions of SARS-CoV-2 and HCoVs S proteins are more commonly expanded in the memory B-cells.
Coronavirus-specific immunity in children and adults
In general, HCoV infections are more frequent in children and become rare with age. This is due to the progressive accumulation or maturation of immunity to HCoVs.
Children exhibit a more adaptable and polyreactive antibody response. There are notable differences in antibody class use and S and N antigen use between children and adults.
The B-cell repertoire reactive with HCoVs and cross-reactive with SARS-CoV-2 is progressively shaped through age due to the frequency and accrued exposure to HCoVs.
The potential function of heterologous immunity
Although antibodies contribute to protective immunity, heterologous immunity may also have a negative impact that can be counterproductive or pathogenic. Heterologous immunity induced by HCoVs or one of the emerging SARS-CoV-2 variants may compromise the individual's response to another SARS-CoV-2 variant. Whether cross-reactive antibodies contribute to pathogenicity is an open question and requires further investigation.
Cross-reactive antibodies that bind to the SARS-CoV-2 S protein can provide some protection through different modes of action. Most commonly, antibodies neutralize the virus and interfere with cellular entry.
Several studies detected little or no neutralizing activity against SARS-CoV-2 in most pre-pandemic samples. However, animal studies have shown that cross-reactive antibodies neutralize other coronaviruses and may potentially contribute to protection.
Does immunological memory protect against coronavirus infections?
The pre-existing immunological memory of HCoVs is unlikely to prevent SARS-CoV-2 infection. However, it may modify its outcome.
The whole population has antibodies against HCoV, yet individuals experience multiple HCoV reinfections over their lifetime. Thus, these cross-reactive antibodies would be unlikely to prevent heterologous infection with SARS-CoV-2.
Even so, epidemiological data suggest that HCoV infection does provide some protection from homologous reinfection and partial protection from heterologous infection, although it is incomplete or short-lived.
Conclusion
SARS-CoV-2 is a bat coronavirus and vaccination against the SARS-CoV-2 S protein should provide a certain degree of protection against other bat coronaviruses that may attempt to cross the species barrier. S2 is highly conserved among animal coronaviruses and between animal coronaviruses and HCoVs. Thus, it can provide cross-reactive immunity against the new emerging variants and other coronaviruses.