Stenoparib, which is a small molecule that is also known as 2X-121, is an inhibitor of mammalian poly (ADP-ribose) polymerases (PARPs). Stenoparib inhibits viral replication by affecting the pathways of the host; thus, it is a host-targeting therapeutic.
 Study: Stenoparib, an inhibitor of cellular poly (ADP-ribose) polymerases (PARPs), blocks in vitro replication of SARS-CoV-2 variants. Image Credit: PHOTOCREO Michal Bednarek / Shutterstock.com
Study: Stenoparib, an inhibitor of cellular poly (ADP-ribose) polymerases (PARPs), blocks in vitro replication of SARS-CoV-2 variants. Image Credit: PHOTOCREO Michal Bednarek / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Stenoparib
There are 46 SARS-CoV-2 variants and only two antiviral drugs, remdesivir and molnupiravir, that are currently approved by the United States Food and Drug Administration (FDA). Remdesivir and molnupiravir affect the activity of the viral ribonucleic acid (RNA)-dependent RNA polymerase (RdRp). In contrast, stenoparib inhibits the host poly (ADP-ribose) polymerase (PARP), thus affecting host pathways to inhibit viral replication.
Remdesivir inhibits viral replication after viral entry into the cell, whereas stenoparib inhibits virus entry and post-entry processes.
Previous in vitro studies have shown that stenoparib inhibits SARS-CoV-2 USA-WA1/2020 and the human coronavirus-NL63 (HCoV-NL63), which is a human seasonal respiratory coronavirus. To this end, this agent suppresses virus multiplication and cell to cell spread in a dose-dependent manner.
Stenoparib inhibits SARS-CoV-2 VOCs
Since stenoparib inhibits a host protein, the antiviral activity of stenoparib should inhibit all variant strains. The current study explored the activity of stenoparib against four SARS-CoV-2 strains including the SARS-CoV-2 67 Germany/BavPat1/2020 wild-type strain (wt), as well as the Alpha, Beta, Gamma SARS-CoV-2 VOCs.
SARS-CoV-2 strains were mixed with serial dilutions of stenoparib and transferred to Vero E6 green monkey kidney cells. The ViroSpot assay was performed to estimate positive staining for the virus.
Stenoparib inhibited virus replication in a dose-dependent manner across all four tested strains of SARS-CoV-2. Interestingly, a higher concentration of stenoparib was required to inhibit the Beta variant, even when used in combination with remdesivir. This may be because the Beta variant replicates rapidly and has a reduced time period between infection and the appearance of the mature virus within the cell.
Stenoparib and remdesivir act synergistically
The researchers of the current study also evaluated the inhibitory action of a combination of stenoparib and remdesivir against the Alpha variant. Vero E6 cells were infected with the Alpha variant, which was followed by an assessment of the activity of stenoparib using the plaque reduction assay. Plaques are areas of dead or destroyed cells that appear as small, clear regions in an infected cell monolayer after staining.
For the analysis, lower doses of stenoparib were combined with the previously reported 50% effective concentration (EC50) of remdesivir. Neither stenoparib nor remdesivir achieved greater than a 50% reduction in plaquing efficiency compared to the infected, untreated cells.
When combined, the drugs acted synergistically and plaque inhibition increased to over 90%. This activity was superior to what was achieved with either drug alone. Notably, this combination did not cause significant cytotoxicity.
Since both remdesivir and stenoparib have two different mechanisms of action, the combined effect of these drugs appears to be synergistic. This provides the potential benefit of minimizing undesirable side effects by reducing individual doses of each drug.
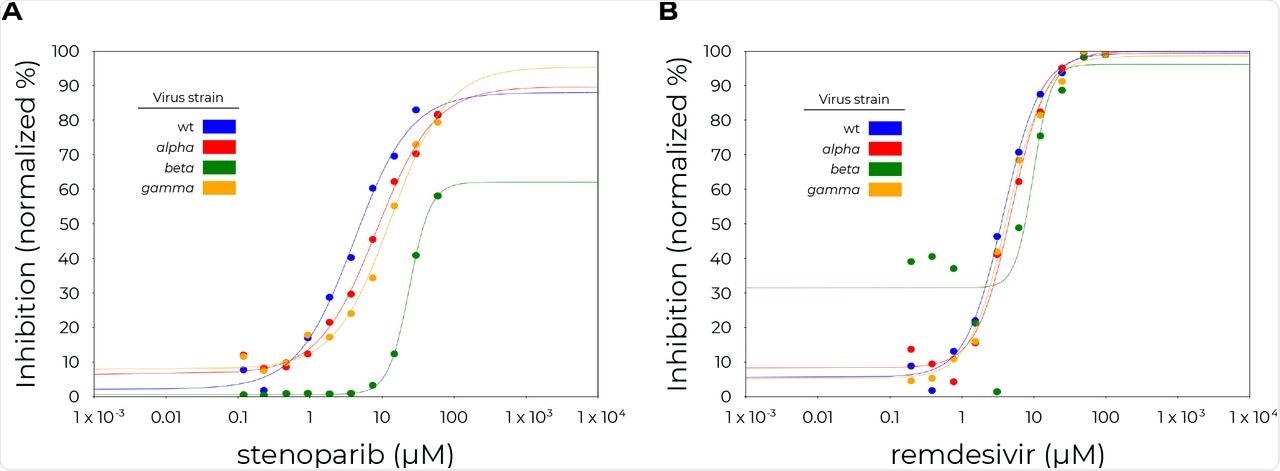 Dose-response curves for stenoparib and remdesivir on SARS-CoV-2 variants. A. stenoparib. B. remdesivir. Vero E6 cells (ATCC CRL-1586) were infected for 20 hours with SARS-CoV-2 wild type (wt) or the indicated variants in the presence or absence of stenoparib or remdesivir as indicated. Results are the average fraction of quadruplicate wells that exhibited positive staining for SARS-CoV-2 (y-axis) vs. the concentration of the inhibitors (x-axis). These data were used to estimate the EC50 of stenoparib and remdesivir. The EC50 values were approximated with the aid of the online calculator from AAT Bioquest (“Quest Graph™ EC50Calculator.” AAT Bioquest, Inc, 26 Oct. 2020, https://www.aatbio.com/tools/ec50-calculator). Viruses; wild-type (wt): BetaCoV/Munich/BavPat1/2020, European Virus Archive Global. Alpha: USA/CA_CDC_5574/2020, BEI Resources, Cat# NR-54011. Beta: hCoV-19/South Africa/KRISP-K005325/2020, BEI Resources. Gamma: hCoV-19/Japan/TY7-503/2021 (Brazil P.1), BEI Resources.
Dose-response curves for stenoparib and remdesivir on SARS-CoV-2 variants. A. stenoparib. B. remdesivir. Vero E6 cells (ATCC CRL-1586) were infected for 20 hours with SARS-CoV-2 wild type (wt) or the indicated variants in the presence or absence of stenoparib or remdesivir as indicated. Results are the average fraction of quadruplicate wells that exhibited positive staining for SARS-CoV-2 (y-axis) vs. the concentration of the inhibitors (x-axis). These data were used to estimate the EC50 of stenoparib and remdesivir. The EC50 values were approximated with the aid of the online calculator from AAT Bioquest (“Quest Graph™ EC50Calculator.” AAT Bioquest, Inc, 26 Oct. 2020, https://www.aatbio.com/tools/ec50-calculator). Viruses; wild-type (wt): BetaCoV/Munich/BavPat1/2020, European Virus Archive Global. Alpha: USA/CA_CDC_5574/2020, BEI Resources, Cat# NR-54011. Beta: hCoV-19/South Africa/KRISP-K005325/2020, BEI Resources. Gamma: hCoV-19/Japan/TY7-503/2021 (Brazil P.1), BEI Resources.
Stenoparib mode of action
PARP enzymes have a role in DNA repair; however, several members of the PARP family have other additional functions. The 18 known human PARPs appear to affect viral replication differentially; while some exhibit proviral activity, others exhibit antiviral activity.
The SARS-CoV-2 nucleocapsid (N) protein is mono ADP ribosylated (MARylated) during infection. The stability of the N protein is essential for viral replication and modulation of the host cell cycle.
Until now, the N protein is the only ADPR target identified in SARS-CoV-2. This modification is prevalent across multiple coronavirus families; thus, ADPR may have a role in regulating virus genome structure.
Remdesivir and molnupiravir are known to inhibit the viral replicon, whereas stenoparib most likely acts through multiple targets. The current study provides a proof of concept that a combination of stenoparib and remdesivir or molnupiravir may be potent at inhibiting SARS-family coronaviruses, including SARS-CoV-2.
Importance of the study
SARS-CoV-2 has caused over 247 million infections and over 5 million deaths worldwide. Even with vaccination, the pandemic continues as newer SARS-CoV-2 variants may exhibit degrees of resistance to vaccination. Thus, there is a need for effective therapeutics.
Stenoparib effectively inhibits replication of SARS-CoV-2 wild-type and variant strains in vitro. A host-targeting drug like stenoparib may be beneficial for COVID-19 patients as a standalone therapy, or in combination with an antiviral drug such as remdesivir or molnupiravir.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zarn, K. E., Jaramillo, S. A., Zapata, A. R., et al. (2021). Stenoparib, an inhibitor of cellular poly (ADP-ribose) polymerases (PARPs), blocks in vitro replication of SARS-CoV-2 variants. bioRxiv. doi:10.1101/2021.11.03.467186. https://www.biorxiv.org/content/10.1101/2021.11.03.467186v1
- Peer reviewed and published scientific report.
Zarn, Katherine E., Sierra A. Jaramillo, Anthony R. Zapata, Nathan E. Stone, Ashley N. Jones, Haley E. Nunnally, Erik W. Settles, et al. 2022. “Stenoparib, an Inhibitor of Cellular Poly (ADP-Ribose) Polymerases (PARPs), Blocks in Vitro Replication of SARS-CoV-2 Variants.” Edited by Philippe A. Gallay. PLOS ONE 17 (9): e0272916. https://doi.org/10.1371/journal.pone.0272916. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0272916.