Older age is a strong risk factor for severe coronavirus disease 2019 (COVID-19) compared to children and younger adults with COVID-19. In addition, older adults infected by SARS-CoV-2 are rarely asymptomatic and experience severe symptoms such as respiratory failure and multi-organ dysfunction that can lead to hospitalization and/or death. Therefore, older adults have been granted priority for receiving COVID-19 vaccines.
 Study: Characterization of the humoral immune response to BNT162b2 in elderly residents of long-term care facilities five to seven months after vaccination. Image Credit: Pordee_Aomboon / Shutterstock.com
Study: Characterization of the humoral immune response to BNT162b2 in elderly residents of long-term care facilities five to seven months after vaccination. Image Credit: Pordee_Aomboon / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Older adults living in long-term care facilities (LTCFs) experience increased exposure to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), increasing their risk of COVID-19-related mortality. During the early pandemic, 30-60% of all COVID-19-related deaths in Europe were attributed to LTCF residents. However, elderly individuals with comorbidities and frailty were underrepresented or excluded from early COVID-19 vaccine trials.
The emergence of new SARS-CoV-2 variants of concern (VOCs), such as the recent Delta variant, and the concerns about waning immunity prompted a discussion on the need for a third booster shot of the COVID-19 vaccine. However, the effect of old age and frailty on immunity against severe disease after vaccination is not fully clear.
The impact of old age and multimorbidity on the immune response to vaccination
A recent study published on the preprint server medRxiv* investigates whether markers of cellular and humoral immunity differ significantly between adults over the age of 75 years and a control group six months after vaccination.
The current study also assessed the effect of medication and multimorbidity on the immune response. The researchers examined the immune response to the BNT162b2 messenger ribonucleic acid (mRNA) vaccine against SARS-CoV-2 five to seven months after receiving two doses of the vaccine.
Most elderly LTCF residents had no detectable antibodies against the Delta variant
All study participants were required to have previously completed their two-dose schedule of the BNT162b2 vaccine at the recommended interval of 21 days. Vaccination was to be completed five to seven months before blood collection.
The first group consisted of LTCF residents who were older than 75 years and did not have any history of prior SARS-CoV-2 infection, which was confirmed by anti-nucleocapsid (N) antibody tests. Whereas the residents were within the age range of 75 to 101 years, the control group of health care workers (HCWs) was aged 18 to 70 years. Notably, the third group of 14 LTCFs was included in the study who had experienced a breakthrough infection.
Out of the 298 SARS-CoV-2-naïve residents, 29 (9.5%) had detectable antibodies against the Delta variant, of which 14 (48.3%) only had a borderline antibody titer of 1:10. Of the 114 HCW controls, 31.6% had detectable neutralizing antibodies.
In the third group of 14 elderly residents, the mean antibody titer was significantly higher compared to the other two groups. Furthermore, 12 (85.7%) of these residents had detectable antibodies against the Delta variant.
This data demonstrates that 90.5% of LTCF residents had no detectable neutralizing antibodies against the dominant Delta variant five to seven months post-vaccination. Additionally, neutralizing antibody titers were higher in those who had a breakthrough infection. These observations suggest that both LTCF residents and HCWs would benefit from a third vaccine shot six months after completing the two-dose regimen.
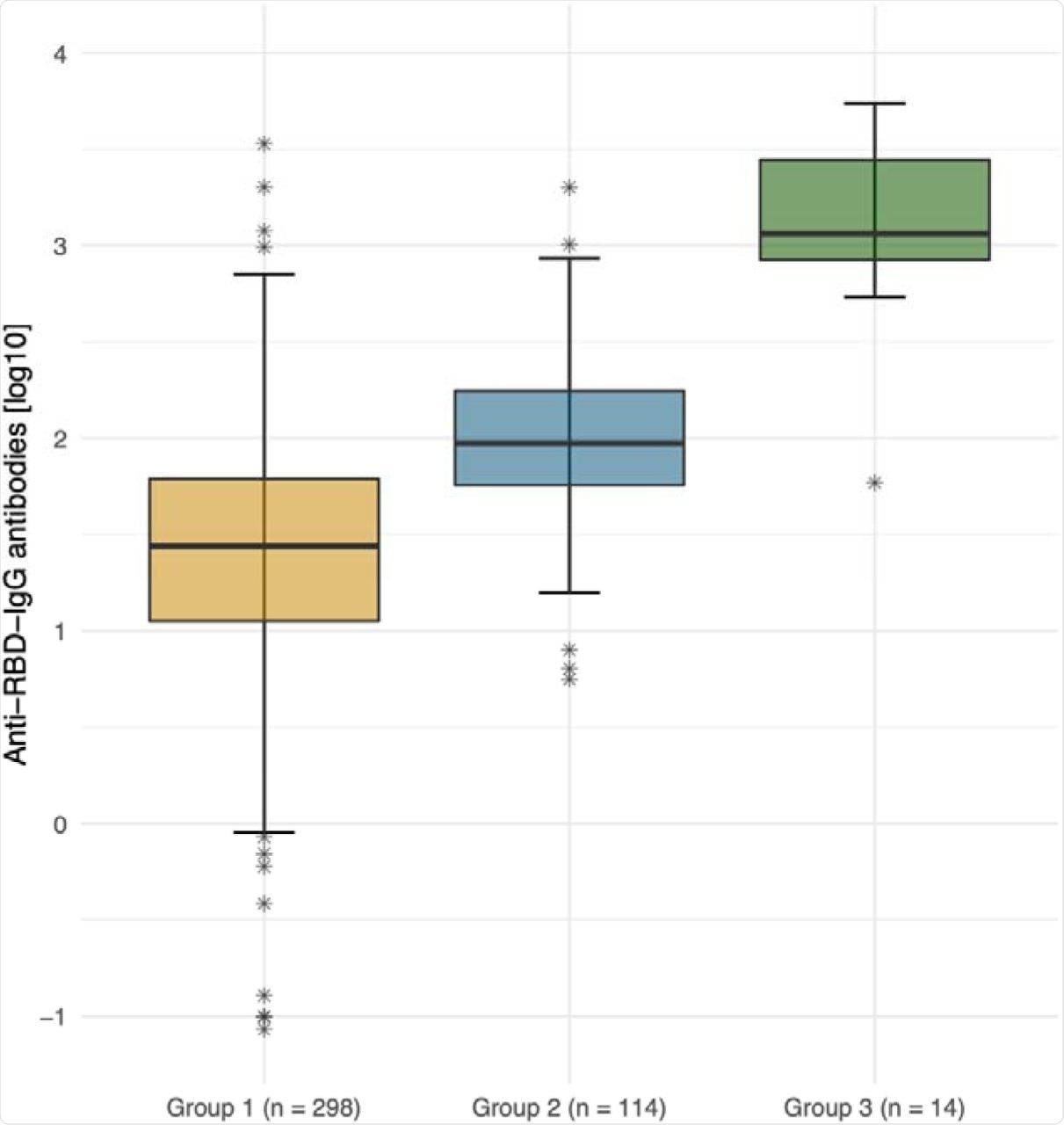 Anti-SARS-CoV-2 spike IgG antibody titers (logarithmic) by group. Logarithmic depiction in boxplots, 95% CI and IQR (25%-75%) of group 1 (residents of LTCFs ≥ 75 years of age; orange), group 2 (HCWs at LTCFs, 18 to 70 years of age; blue), and group 3 (residents of LTCFs after breakthrough infection, green). Stars represent outliers.
Anti-SARS-CoV-2 spike IgG antibody titers (logarithmic) by group. Logarithmic depiction in boxplots, 95% CI and IQR (25%-75%) of group 1 (residents of LTCFs ≥ 75 years of age; orange), group 2 (HCWs at LTCFs, 18 to 70 years of age; blue), and group 3 (residents of LTCFs after breakthrough infection, green). Stars represent outliers.
“A linear decline with age was observed in both the group of residents and HCWs but was more marked in the elderly.”
The results agree with other studies conducted in older adults, although studies using pseudoviruses reported higher neutralization rates. A possible explanation for this might be that this study used an authentic Delta variant isolate, rather than a pseudovirus, for the neutralization assays. Clinical isolates have additional mutations outside the spike region, which impact viral fitness or their sensitivity to antibodies.
A breakthrough infection significantly increased both antibody titers and the number of individuals with detectable neutralizing antibodies against the Delta variant among the elderly participants. This demonstrates that the humoral immune response is recoverable.
According to the authors, markers of the cellular response of the participants are currently being examined and that data may influence the interpretation of the findings.
“In addition to age, immunogenicity and efficacy of vaccines 219 may be influenced and suppressed by several factors, including other medical conditions, and body composition with a very low or high BMI.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Delbrück, M., Hoehl, S., Toptan, T., et al. (2021). Characterization of the humoral immune response to BNT162b2 in elderly residents of long-term care facilities five to seven months after vaccination. medRxiv. doi:10.1101/2021.11.09.21266110. https://www.medrxiv.org/content/10.1101/2021.11.09.21266110v1.full-text.
- Peer reviewed and published scientific report.
Delbrück, Marla, Sebastian Hoehl, Tuna Toptan, Barbara Schenk, Katharina Grikscheit, Melinda Metzler, Eva Herrmann, and Sandra Ciesek. 2022. “Low but Recoverable Markers of Humoral Immune Response to BNT162b2 in Elderly LTCF Residents Five to Seven Months after Two-Dose Vaccination.” Frontiers in Aging 3 (April). https://doi.org/10.3389/fragi.2022.883724. https://www.frontiersin.org/articles/10.3389/fragi.2022.883724.