Coronavirus disease (COVID-19) is highly contagious. It is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which belongs to the betacoronavirus cluster comprising seven members, including SARS and the Middle East respiratory syndrome (MERS).
Methods used to screen and diagnose SARS-CoV-2 infection include detecting SARS-CoV-2 nucleic acid, SARS-CoV-2-specific antibody, and antigen. Antigen-based diagnostic tests are less sensitive than reverse transcription-polymerase chain reaction (RT-PCR) -based tests, but they are faster and more convenient alternatives.
The positive rates of SARS-CoV-2 nucleic acid in different body fluids are variable, indicating a distinct pattern of persistence and clearance of viral RNA in body fluids of COVID-19 patients. Furthermore, the positive rates detected in extra-pulmonary specimens are usually lower than those seen in nasopharyngeal swabs.
It was also found that under certain specific conditions, SARS-CoV-2 might infiltrate from the bloodstream to the kidney parenchyma and may eventually result in renal injury and the urinary shedding of viruses.
Acute kidney injury (AKI) is a common complication among hospitalized patients with severe COVID-19 symptoms despite a low urinary virus RNA positive rate among COVID-19 patients.
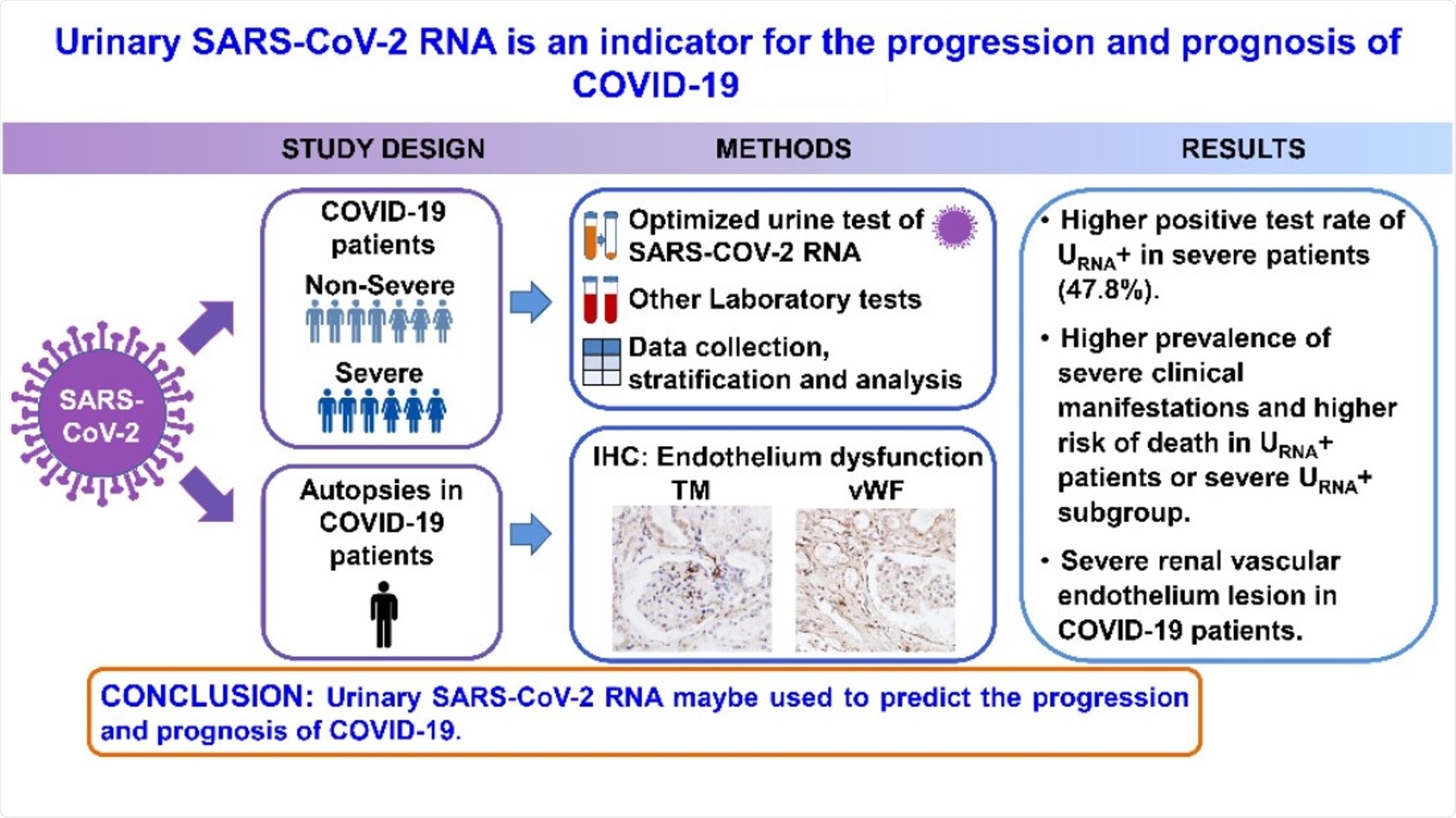
Urinary SARS-CoV-2 RNA Is an Indicator for the Progression and Prognosis of COVID-19
The Study
A new study published in the journal Diagnostics was based on the hypothesis that detecting urinary SARS-CoV-2 nucleic acid––which may result in renal and cardiovascular endothelial destruction to facilitate viral access to the kidney parenchyma—may be used as a specific biomarker to indicate the severity of COVID-19.
The present study included 53 patients diagnosed with COVID-19 at Renmin Hospital of Wuhan University from 31 January 2020 to 18 February 2020. Participants were tested for SARS-CoV-2 nucleic acid in urine samples with quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis.
Based on the results, the patients were divided into two groups – the urinary SARS-CoV-2 negative group (URNA−, 38 cases) and the positive group (URNA+, 15 cases). In addition, a retrospective study was conducted on the patients’ clinical characteristics, pre-existing diseases, and laboratory tests.
The diagnosis of COVID-19 pneumonia was conducted by following the New Coronavirus Pneumonia Prevention and Control Guidance. This research was funded by the National Natural Science Foundation of China and the National Institutes of Health.
Findings
The median age of the patients was 52 years (age-range, 42-66 years). Results of Real-Time Quantitative Reverse Transcription-PCR (qRT-PCR) analysis showed that 38 out of the 53 patients were urinary SARS-CoV-2 negative (URNA−). While the urinary SARS-CoV-2 positive (URNA+) patients were older and more likely to experience chest tightness and shortness of breath. Yet, the latter group showed no significant differences in the gender-based distribution, common symptoms, radiological appearance, metabolic diseases or comorbidities.
Meanwhile, URNA+ patients developed more severe respiratory distress with manifestations of lower arterial oxygen pressure (PaO2) and oxygen saturation (SaO2), as examined by arterial blood gas analysis. In addition, leukopenia and lymphocytopenia were detected more frequently in routine blood tests of URNA+ patients than those in blood tests of URNA− patients
Furthermore, immune profile evaluation identified a more frequent increase of serum C-reactive protein (CRP) and immunoglobulin (Ig)E in URNA+ patients. Additionally, this group had a higher incidence of increased serum levels of alanine aminotransferase (ALT), higher percentage of increased serum aspartate aminotransferase (AST), increased serum myoglobin, lactate dehydrogenase (LDH), blood urea nitrogen (BUN), and decreased estimated glomerular filtration rate (eGFR) than URNA− patients.
Hence, multiple vital organs were more severely affected in URNA+ patients. This group also exhibited markedly lower levels of T cells and T helper (Th) cells in peripheral blood mononuclear cells. The results depicted a correlation of the urinary SARS-CoV-2 RNA with COVID-19 severity and the underlying conditions in COVID-19 patients.
Among the 23 patients with severe disease, 12 were urinary SARS-CoV-2 negative (S URNA−), and 11 of them were URNA+ (S URNA+). The positive rate of urine SARS-CoV-2 RNA was significantly higher in severe patients than that in non-severe patients. Thus, the urine shedding SARS-CoV-2 correlated with the severity of the disease.
It was also found that S URNA+ patients had more comorbidities. This group also had significantly higher levels of IgE and IgG than S URNA− patients, along with a higher risk for death than the severe patients with URNA−.
Additionally, the expression of Thrombomodulin (TM) and von Willebrand Factor (vWF) in interstitial vessels, glomerular and tubules were higher in kidneys from COVID-19 patients who had died compared to those in kidneys from renal carcinoma patients.
Relevance of the Study
The findings of this study indicated that the positivity of SARS-CoV-2 RNA in urine specimens might be used to predict the progression and prognosis of COVID-19. The positivity rate detected was 28.3%, which is higher than the test results on routine urine sample testing.
Therefore, the optimized urine SARS-CoV-2 RNA test method used in this study could improve the positive rate, which may assist in predicting the disease outcome, especially in severe patients. Meanwhile, recovering patients have a limited chance of spreading the virus through urine.
Furthermore, the results underscored a potential association of vascular endothelial damage with virus urine shedding. Hence, detecting SARS-CoV-2 RNA in urine sediments can be a potential biomarker for evaluation and prognosis for patients with COVID-19.
Journal reference:
- Zhang, L., Tian, M., Song, Y., et al. (2021), “Urinary SARS-CoV-2 RNA Is an Indicator for the Progression and Prognosis of COVID-19”, Diagnostics, 11(11), 2089, doi: 10.3390/diagnostics11112089, https://www.mdpi.com/2075-4418/11/11/2089/htm